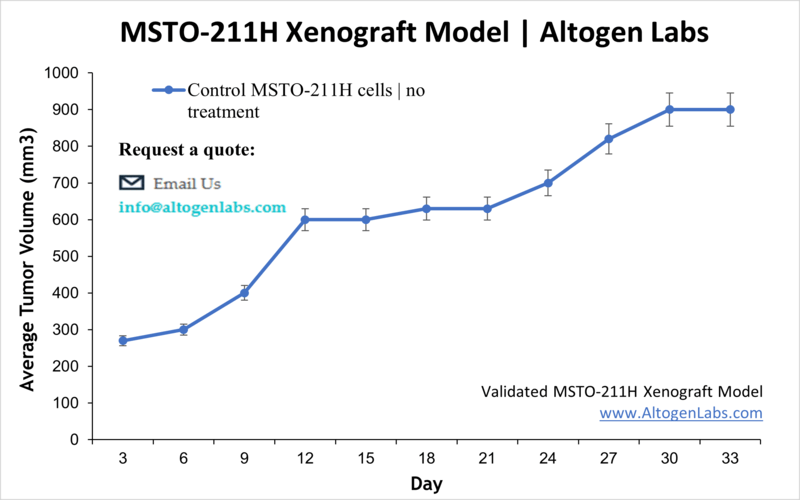
MSTO-211H Xenograft Model
Mesothelioma is a subtype of cancer that affects the mesothelium, a thin tissue layer covering many internal organs including the lungs, chest wall, heart sac, testis sac, and abdomen. Over 80% of mesothelioma cases are due to asbestos exposure. Prognosis is poor, with less than an 8% five year survival rate. The MSTO-211H cell line was derived from the metastatic pleural effusion of a 62 year old Caucasian male with biphasic mesothelioma. The patient received no prior treatment- chemotherapy or radiation. The MSTO-211H model has been used in many mesothelioma research studies. A 2008 Molecular Biology study used the MSTO-211H to investigate a novel gene therapy and virotherapy technique: targeting CREBBP/EP300 (CRI1), a mesothelioma specific gene expression, using CRI1-138, 4x, a recombinant system that is four tandem repeats of the CRI1 promoter with an adenoviral vector expressing a proapoptotic death agonist that interacts with BH3. Results demonstrated this technique successfully has antitumor effects in MSTO-211H xenografts. In 2006 Spugnini et al. published a Clinical Cancer Research article characterizing the solitary and combinatorial effects of piroxicam, a nonsteroidal anti-inflammatory drug, with cisplatin (CDDP). Data reported piroxicam treatment resulted in proliferation inhibition, increased apoptosis, and increased cell cycle arrest in vitro. In vivo reduction in tumor growth, extended survival, upregulated metabolic pathway genes and downregulated RNA processing genes was observed in MSTO-211H xenografts with combination treatment with piroxicam and CDDP, suggesting their potential use in clinical treatment of mesothelioma. Lastly, Nayak et al. (Radiology, 2013) used MSTO-211H cells to evaluate the use of radiolabeled antibodies targeting HER1 and HER2 for MRI of malignant pleural mesothelioma (MPM) in mice. Results demonstrated success with using anti-HER1 radiolabeled antibodies for MPM imaging, which has implications for evaluating stage of cancer as well as monitoring treatment efficacy. The MSTO-211H cell line is used to create the CDX (Cell Line Derived Xenograft) MSTO-211H xenograft mouse model. The MSTO-211H xenograft model has been used to investigate mesothelioma treatments (piroxicam, cisplatin, virotherapy etc).
Basic Study Design
- MSTO-211H cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using flow cytomerty (Guava) or trypan blue exclusion assay (98% of cell viability is required). MSTO-211H cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 106 cells in 150-200 µL of a Matrigel- MSTO-211H cell suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 80-120 mm3 as measured via digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the pre-established treatment schedule.
- Mice weights are measured and recorded 2-3 times weekly; tumors are measured and recorded daily.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNA later reagent, snap frozen in liquid nitrogen or prepared for histology.
