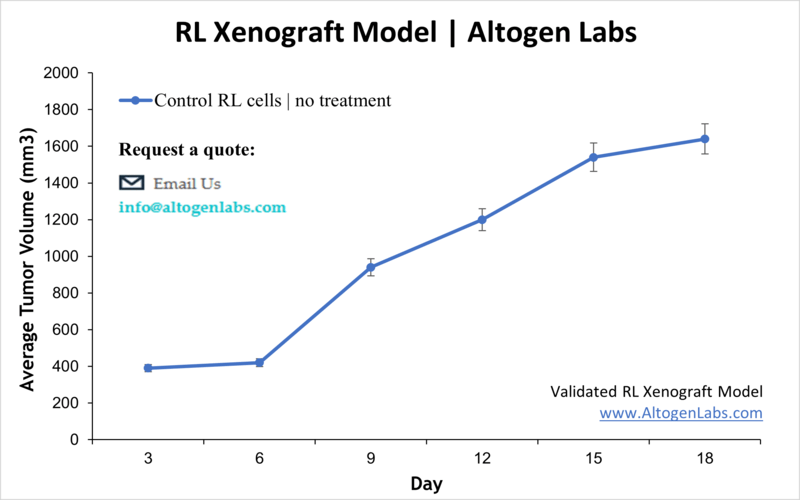
RL Xenograft Model
Non-Hodgkin’s lymphoma (NHL) is a subtype of lymphoma, a cancer characterized by an abundance of abnormal lymphocytes (a white blood cell type). NHL most commonly affects adults and typically originates in lymph nodes or other type of lymph tissue (spleen, bone barrow, thymus, etc). NHL can affect either type of lymphocyte, B cell or T cell, and can be either indolent or aggressive. The specific type diagnosis helps to set an NHL treatment course. The RL cell line was, in 1893, established by Dan L. Longo and Walter J. Urba from the ascites of a 52YO Caucasian male diagnosed with non-Hodgkin’s lymphoma affecting B cells. RL cells have since been used as models for research investigating B cell NHL. A 2013 Blood Cancer Journal article (Decaup et al.) used RL cells as a follicular lymphoma model in order to investigate the differences in response to combination treatment in 2D and 3D cell models. The group created multicellular aggregates of lymphoma cells, or MALC, in order to mimic a 3D culture as seen in tumor special organization. Results demonstrated that 3D models had a more sensitive response to rituximab (RTX, an anti-CD20 monoclonal antibody) and obinutuzuman (GA101, a type II anti-CD20 monoclonal antibody). Rosana et al. (Clinical Cancer Research, 2005) used an RL xenograft as a B-lymphoma model to characterize the antitumor effects of anti-CD74, CD20 or HLA-DR antibodies conjugated to radionuclides that emit low-energy electrons. Results demonstrate that the anti-CD20 antibody with either radiolabeled 111IN or 125I successfully treated xenografted tumors. These data have implications for the treatment of micrometastatic cancer. The RL cell line is used to create the CDX (Cell Line Derived Xenograft) RL xenograft mouse model. The RL xenograft model has been used as a model for studying non-Hodgkin’s lymphoma treatments (immunotherapy using antibody conjugates etc).
Basic Study Design
- RL cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using a trypan blue exclusion assay (98-99% of cell viability is required). RL cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 2×106 cells in 180-200 µL of a Matrigel-RL cells suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 100-150 mm3 as measured by digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the preestablished treatment schedule.
- Mice weights are measured and recorded 2-3 times weekly; tumors are measured and recorded daily.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNAlater, snap frozen in liquid nitrogen (LN2), or prepared for histology.
