H526 SCLC Lung Cancer Xenograft Model: Download ![]()
Lung cancer is primarily developed in the older population (over 65 years old). The chance of developing lung cancer in the US is approximately 1 in 16, with the risk being significantly higher for smokers. Approximately 14 percent of all new cancer diagnoses are lung cancer, which has two primary classifications: small cell and non-small cell lung cancer (NSCLC), of which large cell carcinoma (LCC) is a subtype. LCC cells are undifferentiated and lack characteristics of adenocarcinomas, squamous cell carcinomas, glandular cells or small cell carcinomas. Lung cancer is the deadliest type of cancer and is the number one in cancer related deaths.
The NCI-H526 cell line is derived from a small cell lung carcinoma (SCLC) of a 55-year-old male patient, and is widely used in cancer research to understand the biology of SCLC. H526 cells exhibit a round, suspension-growing morphology typical of SCLC and express neuroendocrine markers such as chromogranin A and synaptophysin. These markers are crucial for studying the molecular characteristics of SCLC and its neuroendocrine features. The cell line allows researchers to investigate key genetic and epigenetic alterations commonly observed in SCLC, such as mutations in the TP53 and RB1 genes. In drug discovery, these cells are employed to assess the effectiveness of chemotherapeutic agents, targeted therapies, and new treatment strategies.
Comparing Cisplatin and Oxaliplatin Responses in H526 Small Cell Lung Cancer Cells
H526 is a small cell lung cancer (SCLC) cell line that serves as a critical model for studying chemotherapeutic responses, particularly to platinum-based drugs like cisplatin and oxaliplatin. Cisplatin, a platinum(II) compound, is a widely used chemotherapeutic agent, but its efficacy is often hindered by drug resistance and severe side effects. Researchers have investigated oxaliplatin, a platinum(IV) prodrug, as a potentially more stable and orally available alternative. In H526 cells, cisplatin primarily induces phosphorylation of p38α MAPK and AMPKα1, triggering a stress response that can influence cell survival and apoptosis. In contrast, oxaliplatin induces a broader and more complex phosphorylation response, affecting multiple signaling proteins associated with drug resistance, such as JNK, GSK-3, STATs, and focal adhesion kinase (FAK). This extensive activation of stress-related pathways suggests that oxaliplatin may paradoxically enhance short-term resistance, limiting its therapeutic potential compared to cisplatin. The study highlights the importance of understanding cellular stress responses in drug development, as excessive activation of survival pathways could undermine the effectiveness of platinum-based therapies. These findings underscore the need for targeted strategies to counteract stress-induced resistance in SCLC treatment.
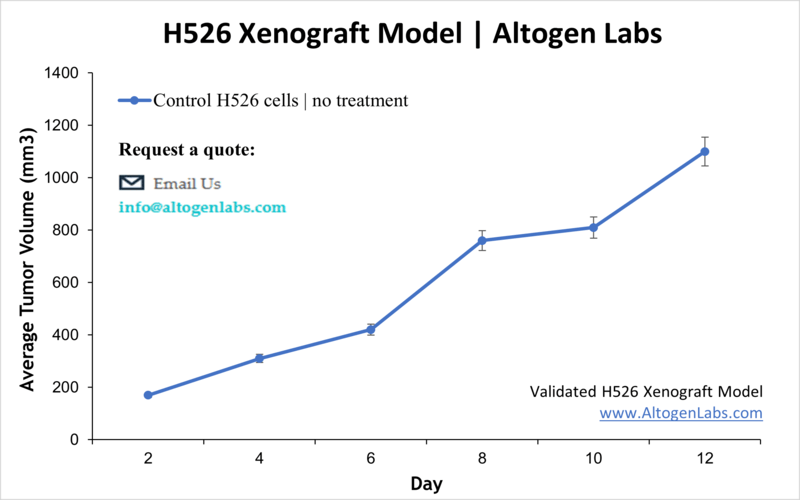
Subcutaneous H526 Lung Cancer Xenograft Model
The H526 subcutaneous model is a widely used xenograft model in cancer research, particularly for studying small cell lung cancer (SCLC). In this model, H526 cells, which originate from a human SCLC tumor, are implanted subcutaneously into immunocompromised mice, typically in the hind legs. By subcutaneously implanting the cells, researchers are provided with an accessible and reproducible way to study tumor growth, drug response, and the underlying biology of SCLC in vivo.
Orthotopic H526 Xenograft Model
The orthotopic NCI-H526 model provides a more physiologically relevant system for studying small cell lung cancer (SCLC) by implanting tumor cells directly into the lungs of immunodeficient mice. This approach closely mimics the tumor microenvironment, allowing for the study of local invasion, tumor-stromal interactions, and disease progression. The model successfully recapitulates key features of human SCLC, including aggressive intrapulmonary growth and metastasis to regional lymph nodes. Histological analysis reveals a highly invasive tumor phenotype with characteristics similar to primary human SCLC. The orthotopic model is particularly valuable for evaluating the efficacy of novel therapies in a setting that better reflects human disease compared to subcutaneous xenografts. It allows researchers to assess drug responses within the lung microenvironment, providing insights into mechanisms of resistance and potential therapeutic vulnerabilities. Additionally, the model facilitates the study of metastatic progression, a hallmark of SCLC, by enabling the observation of tumor dissemination to distant sites.
Targeting NCI-H526 for Complete Tumor Regression
Promiximab-DUBA, a novel CD56-targeting antibody-drug conjugate (ADC), demonstrates potent anti-tumor activity in small cell lung cancer (SCLC). CD56 is highly expressed in SCLC cells, making it a promising therapeutic target. The study highlights the strong efficacy of promiximab-DUBA against the NCI-H526 cell line, with an IC50 value of 0.07 nmol/L, indicating high potency. In vivo, promiximab-DUBA at 5 mg/kg and 10 mg/kg led to complete regression of NCI-H526 tumors without recurrence for 65 days. The ADC maintained strong tumor specificity and internalization properties while minimizing toxicity to normal tissues. Compared to prior CD56-targeted ADCs like Lorvotuzumab Mertansine, promiximab-DUBA exhibits superior efficacy and lower toxicity. The findings support further development of promiximab-DUBA as a promising immunotherapy for SCLC, particularly in tumors expressing CD56, such as NCI-H526. In a xenograft mouse model using H526 tumors, 4C9-DM1 inhibited tumor growth by up to 59% at 5 mg/kg, showing a dose-dependent response. Combination therapy with 4C9-DM1 and carboplatin/etoposide or lurbinectedin resulted in tumor growth inhibition exceeding 85%, suggesting a strong synergistic effect.
Basic Study Design
- NCI-H526 cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using a flow cytometry cell viability assay.
- Each mouse is singly subcutaneously injected into the right flank with 2 x 106 cells in 150-200 µL of a NCIH526 cells.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 100-150 mm3 as measured via digital calipers.
- Animals are randomized into treatment groups.
- Mice weights are measured and recorded 2-3 times weekly.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors and tissues can be stabilized in RNA-Later, snap frozen in LN2 or prepared for histology.
