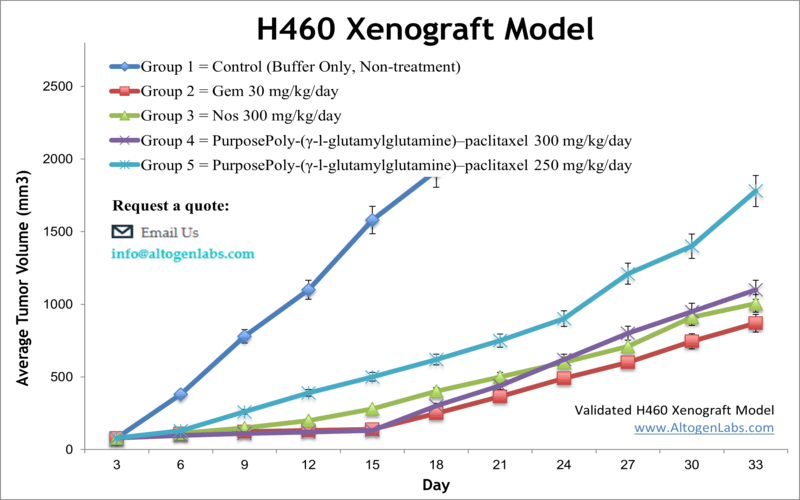
H460 xenograft model
H460 is a human non-small cell lung cancer cell line that was established from the pleural effusion of a patient with large cell carcinoma of the lung. This cell line has been widely used as a model system for studying various aspects of lung cancer biology, including tumor initiation, progression, and drug resistance. Although lung cancer treatment has dramatically improved within the last two decades, it is the primary cause of cancer death in both males and females worldwide. It results in over 158,000 fatalities in the U. S. yearly, as per the American Cancer Society. The H460 epithelial cell line was isolated in 1982 from a male patient with large cell lung carcinoma. It is tumorigenic in nude mice and expresses p53 mRNA. The H460 cell line is negative for neurofilament triplet protein, but stains positively for vimentin and keratin. H460 does not show significant structural DNA abnormalities. A 2016 study published in Molecular Cancer Therapeutics investigated the efficacy of peloruside, a microtubule-stabilizing agent isolated from a New Zealand marine sponge, compared with standard anticancer agents such as paclitaxel, docetaxel, and doxorubicin in the H460 xenograft model. According to the article, peloruside shows an impressive dose-dependent single-agent antitumor activity in the H460 xenograft model. Peloruside is effective in preventing the growth of lung tumors in vivo and could be a clinical candidate for the treatment of lung cancer. A 2010 study by Jafri et al used the H460 cell model to test the efficacy of combining thymoquinone (TQ), a compound found in Nigella Sativa Black Caraway seeds, and cisplatin (CDDP), the most active lung cancer treatment, for lunch carcinoma treatment. Results demonstrated synergism between the combination treatment and was well tolerated in xenograft models, showed decreased tumor volume, downregulated NF-κB expression, inhibition of invasion and pro-angiogenesis cytokines (ENA-78 and Gro-alpha). Overall this supports the use of TQ combination therapy to overcome NF-κB-mediated CDDP resistance. Hsu et al. (2017) published an Environmental Toxicology study using the H460 model for an in vitro and in vivo evaluation of the mechanism of the anti-cancer effects of deguelin, a flavonoid family rotenoid and known Akt inhibitor. Treatmnet with deguelin was shown to decrease tumor growth and trigger cell apoptosis that accompanied an upregulation of pro-apoptotic factors including calcium production, caspase-3, cytochrome c, Bax, Bak, AIR and a decrease in mitochondrial membrane potential. These data support further investigation of deguelin as a potential therapeutic treatment. Lastly, Zhang et al. released a 2017 in Scientific Reports using H460 xenografts modulated to overexpress ABCG2, a transmembrane ATP-binding cassette (ABC) transporter that is responsible for drug efflux and multidrug resistance (MDR) and that is upregulated in many cancers. Results from this model demonstrated correlation of ABCG2 overexpression to drug resistance (such as with mitoxantrone and topotecan) and reversal of this resistance upon treatment with lapatnib, an ABCG2 inhibitor. This establishes the H460/ABCG2-overexpressing model as appropriate for the study of MDR. These genetic alterations are commonly found in lung cancer and contribute to the development and progression of the disease.
H460 Subcutaneous And Orthotopic Model: Download ![]()
Download Altogen Labs H460 Xenograft Model PowerPoint Presentation: ![]()
Orthotopic H460 Xenograft Model
H460 cells are also used in orthotopic lung cancer models to better replicate the tumor’s native microenvironment. These models involve implanting H460 cells directly into the lungs of immunocompromised mice, allowing for the evaluation of tumor growth, progression, and response to therapies. Advanced imaging techniques, such as bioluminescence, are employed to monitor tumor development and metastasis in real time. Orthotopic models have an advantage over traditional subcutaneous models, as they more accurately mimic the tumor in a human, including the potential for metastasis and interactions within the lung microenvironment.
H460 as a Preclinical Model for Lung Cancer Therapy
The H460 lung cancer cell line exhibits strong oncogenic properties, largely due to its side population (SP), which is enriched with stem-like cancer cells. These SP cells demonstrate high tumorigenicity, self-renewal, and significant proliferative capacity, forming anchorage-independent spheres, a hallmark of cancer stem cells. They preferentially express ABCG2, a multidrug resistance protein, and SMO, a key mediator of the Hedgehog (HH) signaling pathway, which regulates cell cycle progression and self-renewal. Additionally, H460 cells are known to exhibit high metabolic activity and resistance to hypoxic conditions, which are hallmarks of aggressive tumors. These cells do not present significant structural DNA abnormalities but have been reported to exhibit altered signaling pathways, such as those involving EGFR, KRAS, or PI3K, which are implicated in cell proliferation and survival. Due to their robust growth characteristics, metastatic potential, and responsiveness to therapeutic agents, H460 cells serve as a reliable preclinical model for evaluating the efficacy of novel cancer therapies and understanding the mechanisms underlying lung cancer oncogenesis.
Targeting NCI-H460 Cells by Inducing G2/M Arrest via Ras/ERK Pathway and p53-p21 Activation
In a study administered by Lv C, et al., published by Marine Drugs journal, researchers examined the effects of asperolide A, a marine-derived tetranorditerpenoid, on the proliferation of NCI-H460 lung carcinoma cells. The compound induced G2/M phase cell cycle arrest by stabilizing the p53-p21 pathway, a mechanism that inhibits cell cycle progression. This arrest was regulated by the Ras/Raf/MEK/ERK signaling pathway, suggesting that ERK activation plays a critical role in the process. In vitro, asperolide A significantly reduced cyclin B1 and CDC2 levels while promoting the activation of p53 and its downstream target, p21, leading to cell cycle disruption. Dominant-negative mutations in Ras blocked these effects, confirming the involvement of the Ras-mediated signaling cascade. In vivo, asperolide A inhibited tumor growth in NCI-H460 xenografts with lower toxicity compared to cisplatin. These findings highlight the therapeutic potential of asperolide A in targeting NSCLC through cell cycle modulation and reduced side effects.
Basic study design
- All flasks are maintained at exponential growth prior to collection.
- The H460 cells are collected by trypsinization. Cell count and viability are then determined by trypan blue exclusion (req 98% viability). Cell suspensions are then adjusted to the density required for inoculation.
- One million cells (volume of 0.1-0.2 mL) of the H460 cell suspension (+ matrigel solution) is subcutaneously injected into the flank of the hind leg per mouse (NOD/SCID or athymic BALB/C, 11-12 weeks old).
- Injection sites are monitored for tumor establishment. Digital calipers are utilized for tumor measurement until tumors reach average sizes of 80-120 mm3.
- After sorting into treatment cohorts (randomization), the test compound is administered following the treatment schedule.
- Daily tumor measurements are recorded, along with mouse weights 3 times weekly.
- Upon reaching the predetermined tumor size limit (or 2,000 mm3), the mice are euthanized.
- As defined for termination of the study, a necropsy is performed.
- Tumors are removed and weighed, and then documented by digital imaging.
- Specified tissues are removed by performing standard gross necropsies.
- Tumors and tissues are snap frozen, stabilized in RNA-Later reagent, prepared for histology, or a nucleic acid isolation performed for genetic analysis.
Get Instant Quote for
H460 Xenograft Model
Altogen Labs provides an array of laboratory services using over 120 CDX and PDX models. Animal handling and maintenance at the Altogen Labs facility is IACUC regulated and compliant to GLP guidelines. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the H460 xenograft model:
- H460 Tumor Growth Delay (TGD; latency)
- H460 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- H-460 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing known chemotherapy drugs
- Imaging studies: Fluorescence-based whole body imaging
