Non-small cell lung cancer (NSCLC) is one a common form of lung cancer that exhibits significant genetic diversity and complex treatment challenges. Xenograft models, developed by transplanting human NSCLC tumors into immunodeficient mice, replicate the tumor’s biological characteristics and microenvironment. These models are vital for exploring cancer progression, uncovering therapeutic vulnerabilities, and evaluating the potential of new treatments, ultimately driving progress in personalized cancer therapy. NCI-H1703 xenograft model is an important research tool in lung cancer studies and testing novel anti-cancer medicines.
H1703 Xenograft Model: Download ![]()
NCI-H1703 Cell Line
The NCI-H1703 cell line is a human non-small cell lung cancer (NSCLC) model derived from a stage I lung squamous cell carcinoma in a 54-year-old Caucasian male smoker. This cell line is characterized by its squamous histology and harbors genetic alterations commonly associated with lung cancer, making it a valuable tool for studying tumor biology and molecular pathways involved in cancer progression. H1703 cells are particularly useful for investigating signaling pathways, such as the platelet-derived growth factor receptor (PDGFR) pathway, due to their amplification of the PDGFRα gene. Researchers frequently utilize this model to evaluate the efficacy of targeted therapies and explore mechanisms of drug resistance. Its relevance to smoking-related lung cancer further enhances its utility in studying carcinogen-induced tumorigenesis and identifying novel therapeutic strategies.
Altogen Labs Subcutaneous and Orthotopic H1703 Xenograft Model
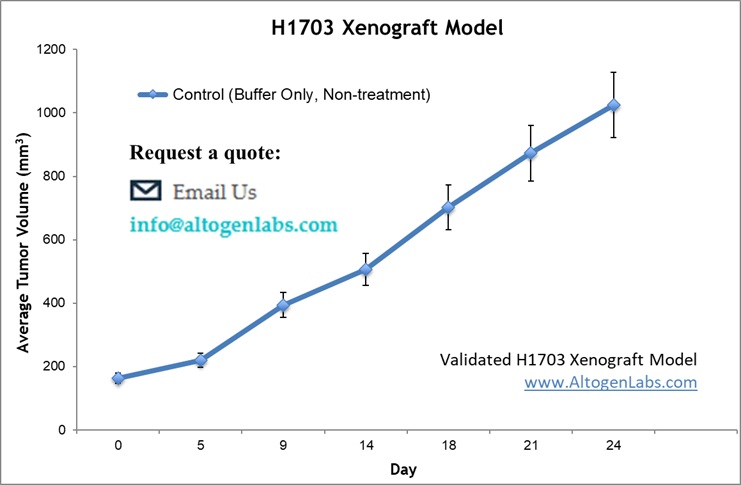
H1703 cells are cultured to the exponential growth phase and suspended in a 1:1 mixture of complete growth medium and Matrigel solution. A 100 µL suspension containing 2 – 5 × 10⁶ cells is subcutaneously injected into the flank above the hind flank of immunocompromised mice, with one tumor established per mouse. Tumor growth is monitored bi-weekly using calipers, allowing tumors to reach ~200-300 mm³ for imaging and biodistribution studies or ~100–150 mm³ before initiating therapeutic interventions. Mice are randomly assigned to control and treatment groups to evaluate the effects of preclinical therapies. Throughout the study, tumor volume, body weight, and general health are systematically recorded, and treatment response is assessed through tumor size reduction, imaging analyses, and post-mortem examination.
The subcutaneous H1703 xenograft model involves the implantation of H1703 tumor cells, derived from human lung squamous cell carcinoma, into immunocompromised mice to study non-small cell lung cancer (NSCLC). These models are established by injecting tumor cells under the skin, enabling straightforward and reproducible monitoring of tumor growth and therapeutic responses. Additionally, it serves as a valuable platform for investigating the molecular mechanisms underlying tumor progression and the role of specific genes or signaling pathways in cancer biology. The subcutaneous location facilitates non-invasive measurement of tumor volume, supporting longitudinal studies on treatment efficacy. Widely used in preclinical research, this model provides critical insights into NSCLC while complementing more complex orthotopic and metastatic models for translational studies.
Orthotopic lung cancer models are critical for accurately studying non-small cell lung cancer (NSCLC) progression, metastasis, and therapeutic response in a physiologically relevant microenvironment. This protocol involves the direct implantation of human NSCLC cell lines, such as H1299 and A549, into the lung tissue of immunocompromised mice to facilitate tumor establishment and growth. The method requires careful preparation of cell suspensions and precise intrapulmonary inoculation under sterile conditions. Tumor progression is monitored through imaging or histological analysis, with tumors possibly metastasizing to contralateral lung lobes and mediastinal lymph nodes. This model closely mirrors human lung cancer pathology, making it invaluable for preclinical evaluation of cancer biology and potential therapies.
Oncogenic Characteristics of the H1703 Human Lung Carcinoma Cell Line
The H1703 cell line is a human lung carcinoma cell line that exhibits several oncogenic characteristics critical for cancer progression. It harbors mutations in key tumor suppressor genes and oncogenes, leading to the activation of various signaling pathways involved in cell proliferation, survival, and metastasis. Notably, the presence of mutated TP53, a hallmark of many cancers, contributes to impaired DNA repair and resistance to apoptosis. The H1703 cells also show overexpression of receptor tyrosine kinases like EGFR, promoting increased cell division and migration. Additionally, alterations in the PI3K/AKT and MAPK pathways are frequently observed in this cell line, enhancing cell growth and survival. These oncogenic features make H1703 an important model for studying lung cancer biology and potential therapeutic interventions.
The H1703 xenograft model provides an excellent platform for evaluating therapeutic agents targeting lung cancer in preclinical studies. Key experimental endpoints such as Tumor Growth Delay (TGD) and Tumor Growth Inhibition (TGI) offer valuable insights into treatment efficacy. The model allows for flexible dosing regimens, including intravenous, intratracheal, intraperitoneal, intratumoral, and oral gavage administration, facilitating tailored treatment protocols. For metastasis studies, alternative cell engraftment sites, such as orthotopic or tail vein injections, are applicable. Comprehensive analyses include tumor immunohistochemistry, survival assessments, gross necropsy, and histopathology, ensuring robust evaluation of therapeutic responses. The model is particularly useful in assessing the efficacy of novel targeted therapies and their mechanisms of action in lung cancer treatment.
Targeting DDR2 Mutations in Lung Squamous Cell Carcinoma
In a study conducted by Hammerman PS, et al., published by Cancer Discovery journal, researchers investigated the role of DDR2 kinase gene mutations in squamous cell carcinoma (SCC) of the lung and their potential as therapeutic targets. Sanger sequencing identified DDR2 mutations in 3.8% of SCC cases, suggesting these alterations as oncogenic drivers. Cell lines with DDR2 mutations demonstrated selective sensitivity to the tyrosine kinase inhibitor dasatinib, which effectively inhibited proliferation and induced apoptosis. In vivo xenograft models further validated dasatinib’s efficacy in reducing tumor growth. Functional assays revealed that DDR2 mutations promoted oncogenic transformation, which was reversed by dasatinib treatment. Additionally, a clinical case demonstrated a radiographic response in a lung squamous cell carcinoma (SCC) patient with a DDR2 mutation treated with a combination of dasatinib and erlotinib. These findings highlight DDR2 as a promising therapeutic target and support the repurposing of FDA-approved drugs like dasatinib for treating lung SCC.
Efficacy of JS-K in H1703 Models
A study conducted by Maciag AE, et al., in The Journal of Pharmacology and Experimental Therapeutics, found that JS-K demonstrated a strong inhibitory effect on a subset of human non-small cell lung cancer (NSCLC) cell lines. JS-K significantly inhibited tumor growth in H1703 xenografts, reducing growth by 75%. Sensitivity to JS-K correlated strongly with the baseline levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS), with high ROS/RNS levels increasing drug efficacy. JS-K treatment induced oxidative and nitrosative stress, leading to glutathione depletion, mitochondrial membrane permeabilization, and cytochrome c release, resulting in apoptosis. The antioxidant N-acetylcysteine (NAC) blocked JS-K’s effects, while the pro-oxidant BSO enhanced its cytotoxicity in H1703 cells. Additionally, peroxiredoxin 1 (PRX1) levels were inversely correlated with ROS/RNS levels and drug resistance, suggesting that PRX1 expression may predict JS-K sensitivity. Treatment also inhibited manganese superoxide dismutase, leading to increased mitochondrial superoxide and DNA damage. These study presents JS-K as a promising therapeutic for lung cancers with high oxidative stress, with PRX1 levels potentially serving as a biomarker for patient selection.
