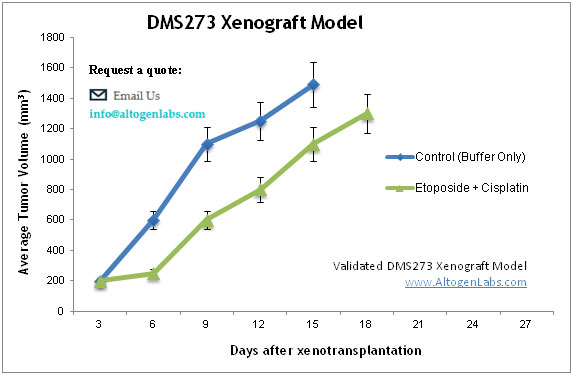
DMS-273 Xenograft Model
DMS273 Lung Cancer Model: Download ![]()
There are over 234,000 new lung cancer cases diagnosed yearly in the United States with over 154,000 annual deaths attributed to the disease. Lung cancer is mostly diagnosed in patients over 65 years of age, with the risk being much higher for those who are smokers. Non-small cell lung cancer (NSCLC) and small cell lung cancer are the primary types. Small-cell carcinoma of the lung is highly malignant and generally displays faster doubling times and metastasis development. The DMS-273 cell line was first established in 1978 by Dr. G D Sorenson of the Lebanon Dartmouth Medical School from the pleural effusion of a previously-treated 50YO Caucasian female patient with small cell lung cancer. DMS273 cells have since been used in many liver cancer studies. In 1997, Yamori et al. (Japanese Journal of Cancer Research) used various lung cancer lines, including DMS273, to examine the efficacy of proposed anti-tumor drug paclitaxel. Today, paclitaxel is a well-known lung, breast, Kaposi sarcoma and lung chemotherapy treatment. In a Cancer Science study, Shuichi et al. (2015) used the DMS273 model to establish a GFP-labeled subline and generated a xenograft via orthotopic transplantation in order to observe metastasis patterns of this small-cell lung cancer model. Using this technique, the group isolated a bone metastasis subline termed G3H and indicated that hepatocyte growth factor and MET signaling pathways greatly contributed to invasion activities. Finally, in a 2006 Nature article, Masuda et al. used the DMS-273 xenograft model in examining the antitumor efficacy of kigamicin D, an agent which targets the ability of cancer cells to tolerate nutrient deprivation. Results demonstrated that while kigamicin D was effective against pancreatic xenografts, it only showed weakly antitumor effects in other models such as DMS-273 and murine syngeneic tumors while in IMC-carcinoma tumor growth actually increased. This data suggests a possible beneficial immune response and highlights the importance of testing agents against a spectrum of cancer types. The DMS273 cell line is used to create the CDX (Cell Line Derived Xenograft) DMS273 xenograft mouse model. The DMS273 xenograft model has been used as a model for studying novel therapies and combination treatments in the highly metastatic small-cell lung cancers.
DMS273 Orthotopic Model Mimics Human SCLC
In an orthotopic transplantation model, DMS273 cells implanted into the lungs of nude mice demonstrated significant metastatic activity, forming secondary tumors in the bone, brain, lymph nodes, kidney, and adrenal gland, mimicking the metastatic pattern observed in human SCLC patients. A GFP-labeled DMS273 subline, termed G3H, was derived from a bone metastasis and exhibited enhanced metastatic ability, with increased hepatocyte growth factor (HGF) expression and activation of the HGF/MET signaling pathway, which promotes tumor invasion and survival. Pharmacological inhibition of MET significantly reduced distant metastases, highlighting the role of this pathway in disease progression. The DMS273 model provides a valuable tool for studying SCLC metastasis and evaluating targeted therapies, particularly those aimed at the HGF/MET axis.
DMS273 Metastatic Model: Studying SCLC Metastasis
The DMS273 metastatic model is a preclinical xenograft system used to study the metastatic progression of small cell lung cancer (SCLC). In this model, DMS273 cells, a human-derived SCLC cell line, are implanted into immunocompromised mice through orthotopic transplantation into the lungs. This setup allows the tumor to replicate the metastatic behavior observed in human SCLC, with secondary tumors forming in distant organs such as the bone, brain, lymph nodes, kidney, and adrenal gland. A GFP-labeled subline, G3H, derived from a bone metastasis, exhibits enhanced metastatic potential, highlighting the role of the HGF/MET signaling pathway in promoting invasion and survival. Pharmacological inhibition of the MET receptor has been shown to significantly reduce distant metastases, underscoring the therapeutic potential of targeting this pathway. The DMS273 metastatic model provides a valuable tool for evaluating therapies aimed at halting metastasis and exploring the underlying mechanisms of disease spread. It also offers an opportunity for drug testing that addresses both primary tumor growth and metastatic dissemination.
Case Study: Targeting HGF/MET in DMS273 Enhances Anti-Metastatic Therapy in SCLC
In a study conducted by Nagel R, et al., published by Molecular Cancer Therapeutics journal introduces a novel metastatic model of small-cell lung cancer (SCLC) using the human DMS273 cell line, which was orthotopically transplanted into nude mice. The GFP-labeled DMS273 cells displayed significant metastatic activity, forming lesions in distant organs such as bone, kidney, and brain, mirroring clinical SCLC metastasis. A highly metastatic subline, G3H, was derived from a bone metastasis, exhibiting increased hepatocyte growth factor (HGF) expression, which promoted cell motility and invasion via the HGF/MET signaling pathway. Pharmacological inhibition of MET using PHA665752 and ARQ-197 significantly reduced metastatic spread, validating the role of HGF/MET in SCLC progression. Importantly, DMS273-GFP and G3H cells retained sensitivity to cisplatin, but metastasis incidence was notably reduced upon treatment, suggesting the model’s utility in evaluating metastatic interventions. This model outperforms previous SCLC models in replicating distant metastasis, making it a valuable tool for investigating novel therapies targeting SCLC dissemination.
Additional Case Study: DMS273 Tumors and Therapeutic Approaches
DMS273 is a highly aggressive small-cell lung cancer (SCLC) subtype known for its rapid proliferation and metastatic potential. In a study by Szot C, et al., published by Journal of Clinical Investigation, researchers explored the efficacy of a novel antibody-drug conjugate (ADC) targeting TEM8, a protein broadly expressed in tumor stroma, including fibroblasts, endothelium, and pericytes. DMS273 tumors responded significantly to TEM8-ADC therapy, leading to tumor regression and, in some cases, complete eradication. The ADC selectively delivered a potent cytotoxic payload to the tumor microenvironment, exploiting the unique stromal composition of SCLC. Notably, the TEM8-ADC demonstrated superior antitumor activity against DMS273 compared to other treatments, highlighting its potential as a targeted therapy for aggressive lung cancers. Additionally, genetic disruption of TEM8 in DMS273 cells reduced ADC effectiveness, confirming TEM8’s role in mediating drug response. Importantly, TEM8-ADC therapy was well tolerated in preclinical models, with minimal systemic toxicity. These findings underscore TEM8 as a promising therapeutic target for treating metastatic SCLC, particularly in highly invasive subtypes like DMS273.
DMS273 SCLC Model: Uncovering Oncogenic Drivers and Metastatic Potential
DMS273 is a highly aggressive variant of small-cell lung cancer (SCLC) characterized by its rapid proliferation, high metastatic potential, and dependence on oncogenic signaling pathways such as c-MYC and HGF/MET. Unlike classic SCLC subtypes, DMS273 exhibits enhanced motility and invasion, leading to frequent metastases in vital organs, including the bone, brain, and kidney. The overexpression of c-MYC in DMS273 contributes to uncontrolled cell growth, metabolic reprogramming, and resistance to standard therapies. Additionally, its autocrine activation of the HGF/MET pathway further amplifies its invasive properties, making it a challenging target for treatment. Studies using orthotopic transplantation models have demonstrated that DMS273 faithfully recapitulates the metastatic behavior observed in SCLC patients, making it a valuable model for drug discovery. Notably, while DMS273 retains some sensitivity to cisplatin, its metastatic dissemination remains a major obstacle, necessitating novel therapeutic strategies. Targeted inhibitors of MET and c-MYC have shown promise in preclinical studies, highlighting the importance of disrupting these key oncogenic drivers. Understanding the molecular mechanisms governing DMS273’s aggressive phenotype will be crucial in developing more effective treatment options for SCLC.
