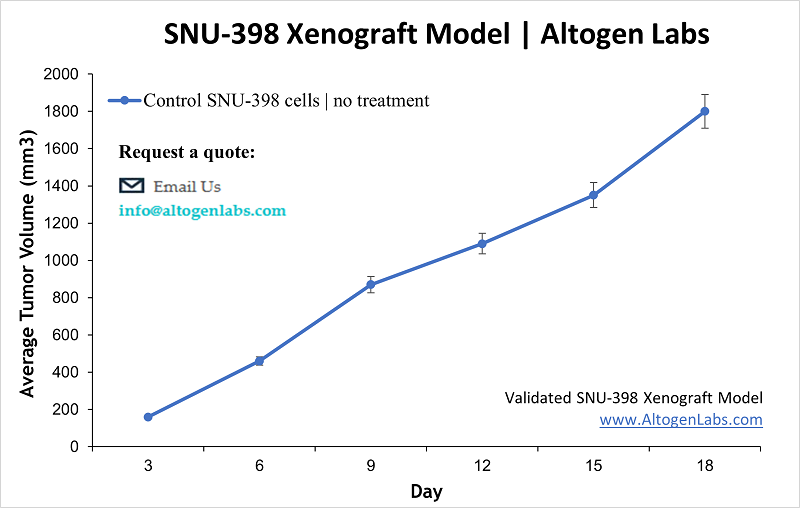
SNU398 Xenograft Model
SNU-398 is a human liver cancer cell line that was established from a patient with a primary hepatocellular carcinoma (HCC), which is the most common type of liver cancer. The SNU-398 cell line has been widely used as a model system to study the biology of liver cancer. Liver cancer has an average 5-year survival rate of around 18% in the United States, with over 30,000 deaths annually and men being three times more likely to develop the disease than women. There are several types of liver cancer defined by the type of cell from which it originates. Hepatitus B and C viruses are the most common cause of liver cancer, and symptoms often include skin yellowing, painful or distended abdomen, easy bruising and weakness. The SNU-398 cell line was first isolated by J.-G. Park in 1990 from a Korean patient diagnosed with hepatocellular carcinoma after the following treatments: 1) lipoidal treatment via transcathetar arterial embolization 2) combination therapy with doxorubicin and mitomycin-C. SNU-398 cells have since been used in many liver cancer studies. A 2008 Cancer Research article by Rodon et al. used the SNU-398 xenograft model to test the efficacies of combination therapy with drugs targeting surface receptors (EGFR and VEGF) and intracellular molecules (angiogenesis or apoptosis). The group used combinations of sorafenib, bevacizumab and cetuximab with inhibitors of MEK, BCL-2 and mTOR; results demonstrated that the combinations of AZD6244 or temsirolimus with sorafenib caused the most tumor growth inhibition and regression with the least toxicity. In 2018, Matsuki et al. published a Cancer Medicine article using SNU-398 xenografts to study the mechanism and effects of the multiple receptor tyrosine kinase inhibitor levatinib on unresectable hepatocellular carcinoma (uHCC). Data demonstrated that in uHCC, levatinib treatment inhibits tumor fibroblast growth factor and angiogenesis pathways as evidenced by decreased microvessel density and suppression of FRS2 and Erk1/2 phosphorylation. Lastly, Schmidt et al. used the SNU-398 model in their 2016 International Journal of Cancer study of S2 cells, an HCC molecular subclass defined by the secretion of E-cadherin, c-myc and AFP and the overexpression of FGFRs. Results confirmed that this subclass is especially sensitive to the FGFR inhibitors BGJ398 and AZD4547, of which the anti-proliferative effects are mediated by MAPK signaling. The SNU-398 cell line is used to create the CDX (Cell Line Derived Xenograft) SNU-398 xenograft mouse model. The SNU-398 xenograft model has been used as a model for studying novel therapies and combination treatments for late stage hepatocellular carcinoma.
Basic Study Design
- SNU-398 cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using MTT or a trypan blue exclusion assay (98% of cell viability is required). SNU-398 cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 106 cells in 150-200 µL of a Matrigel-SNU398 cells cell suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 100-150 mm3 as measured via digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the preestablished treatment schedule.
- Mice weights are measured and recorded 2-3 times weekly; tumors are measured and recorded daily.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNAlater, snap frozen in LN2 or prepared for histology (10% NBF formalin).
