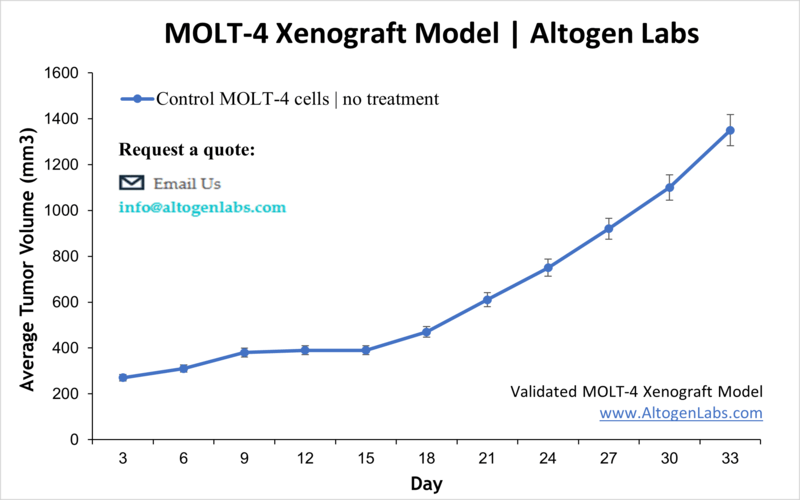
MOLT4 Xenograft Model
Leukemia describes the group of cancers that affect blood-forming tissues, which includes bone barrow. Many leukemias are slow growing although there are many types and treatments depend on the subtype. Aggressive leukemias usually are treated with some combination of chemotherapy, stem-cell transplant, and radiation. There is a lot of room for improvements on current therapies for leukemia. The MOLT-4 cell line are T-lymphoblasts isolated from a 19 year old patient in relapse diagnosed with acute lymphoblastic leukemia. MOLT-4 cells have since been used in many leukemia studies. In 2015 Chao et al. published a Journal of Hemoatology & Oncology article using the MOLT-4 xenograft model to test the combination therapy of vincristine, a microtubule-depolymerizing agent, and vorinostat (suberylanilide hydroxamic acid, a pan-histone deacetylase inhibitor. Results suggested that the combination of suberylanilide hydroxamic acid (SAHA) with vincristine modulated microtubule dynamics through a HDAC6-mediated pathway and resulted in cell cycle arrest and increased cytotoxicity. Pikman et al. (2017) released a Clinical Cancer Research paper utilizing the MOLT-4 xenograft model to test combinations of chemotherapy with CDK4/6 inhibition, which is relevant to patients requiring additional therapy after relapse or refractory disease. They chose this method as Cyclin D3 (CCND3) as well as CDK6 are overexpressed in T-cell acute lymphoblastic leukemia. Results demonstrated that LEE011, a CDK4/6 inhibitor, after being screened against a drug panel, produced the greatest anticancer activity when combined with mTOR inhibitors and corticosteroids. The MOLT-4 cell line is used to create the xenograft mouse model. The MOLT-4 xenograft model has been used as a model for identifying potential leukemia therapies and more specifically for patients in relapse.
Basic Study Design
- MOLT-4 cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using a trypan blue exclusion assay (98% of cell viability is required). MOLT-4 cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 106 cells in 100 µL of a Matrigel-MOLT4 cell suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 120-160 cubic mm as measured by digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the pre-established treatment schedule.
- Mice weights are measured and recorded 3 times weekly; tumors are measured and recorded daily.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNAlater, snap frozen in LN2 or prepared for histology.
