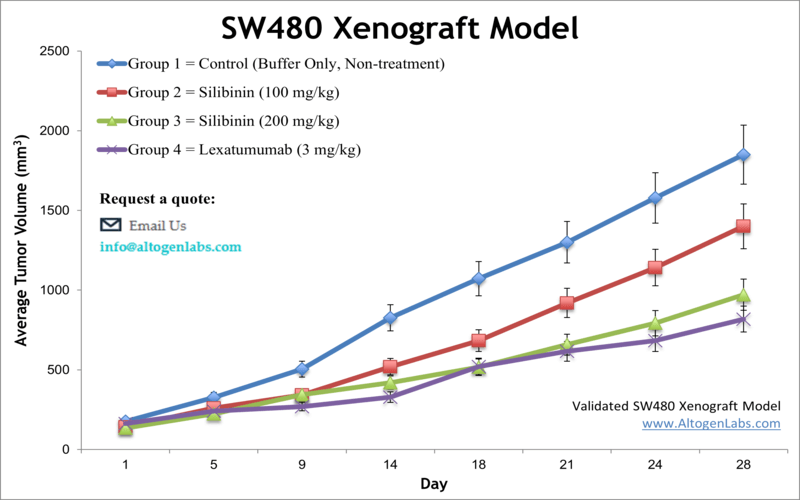
SW480 xenograft model
Download Altogen Labs SW480 Xenograft Model PowerPoint Presentation: ![]()
SW480 Cell Line
The SW480 cell line, derived from a primary adenocarcinoma of the colon, is a widely utilized in vitro model in colorectal cancer research due to its well-characterized molecular profile and clinical relevance to early-stage disease. These epithelial cells harbor pathogenic mutations in KRAS (G12V), APC, and TP53, rendering them a representative model of canonical colorectal tumorigenesis involving dysregulated Wnt/β-catenin signaling and impaired apoptotic pathways. SW480 cells have been instrumental in delineating mechanisms of therapeutic resistance, particularly to 5-fluorouracil and oxaliplatin, and serve as a platform for evaluating the roles of non-coding RNAs, including HOTAIR and miR-21, in promoting tumor cell proliferation, invasion, and survival. Despite extensive characterization in monoculture systems, key gaps remain regarding the influence of the tumor microenvironment, including cancer-associated fibroblasts and immune interactions, on SW480 behavior and drug responsiveness.

SW480 Subcutaneous Xenografts in Colorectal Cancer Research
Subcutaneous xenograft transplantation is a widely adopted preclinical approach for studying colorectal cancer, providing a reproducible and accessible platform for in vivo evaluation of tumor growth and therapeutic responses. The SW480 cell line, derived from a primary colon adenocarcinoma, is frequently used in these models due to its well-characterized mutations in APC, TP53, and KRAS, and its consistent tumor-forming capacity in immunodeficient mice. SW480-derived xenografts are particularly valuable for assessing drug resistance mechanisms, especially to chemotherapeutic agents such as 5-fluorouracil and oxaliplatin, reflecting clinically relevant treatment challenges. These tumors typically display histopathological features of epithelial adenocarcinomas and exhibit moderate growth kinetics, making them suitable for long-term studies involving tumor volume tracking, pharmacological intervention, and histological analysis.
Recent research has expanded the utility of SW480 xenografts by incorporating elements of the tumor microenvironment, including co-injection with cancer-associated fibroblasts or extracellular matrix components, to study stromal influence on signaling pathways and drug sensitivity. Additionally, these models have enabled the investigation of targeted therapies directed at Wnt, MAPK, and PI3K pathways, revealing connections between oncogenic mutations and therapeutic vulnerability. While the lack of immune components and anatomical relevance limits their translational capacity, subcutaneous SW480 xenografts remain foundational to preclinical oncology due to their consistency, scalability, and capacity for mechanistic exploration. As xenograft models evolve to better reflect tumor complexity, the SW480 subcutaneous system continues to play a central role in advancing our understanding of colorectal cancer biology and informing the development of more effective treatment strategies.
Orthotopic SW480 Xenografts for Colorectal Cancer Modeling
Orthotopic xenograft transplantation represents a critical advancement in preclinical modeling of colorectal cancer by allowing tumor cells to be implanted into their site of origin within the host animal. In contrast to subcutaneous models, orthotopic transplantation more accurately recapitulates the tumor microenvironment, including local tissue architecture, stromal interactions, and organ-specific factors that influence tumor behavior. When applied to the SW480 cell line, orthotopic models offer an opportunity to study early-stage colorectal cancer progression in a physiologically relevant setting. The orthotopic model using SW480 cells is particularly valuable for examining tumor-host interactions that regulate growth kinetics, angiogenesis, and immune modulation. Placement of tumor cells into the colonic or rectal wall facilitates access to native extracellular matrix components and local vasculature, which can significantly influence gene expression profiles and treatment response. This model also permits longitudinal monitoring of disease progression and evaluation of therapeutic interventions under conditions that closely mimic human pathology. While orthotopic transplantation requires technical precision and often involves longer tumor latency periods compared to ectopic models, the increased biological fidelity justifies its use in mechanistic studies and translational research. Incorporating SW480 into orthotopic frameworks enhances the relevance of preclinical findings and strengthens the predictive value of experimental outcomes for informing clinical strategies in colorectal cancer.
Modeling Metastasis with SW480 Xenograft Transplants
Metastatic xenograft transplantation provides a critical platform for investigating the biological mechanisms that drive colorectal cancer dissemination and for evaluating novel anti-metastatic therapies. Although the SW480 cell line originates from a primary colorectal adenocarcinoma and is traditionally considered less aggressive in standard subcutaneous models, it can be adapted to study metastatic behavior when introduced into organ-specific environments or modified to mimic advanced disease states. These models are essential for replicating the multi-step cascade of metastasis, including local invasion, intravasation, circulation, extravasation, and colonization of distant organs. When used in appropriate transplantation settings, SW480 xenografts allow researchers to assess tumor cell plasticity, microenvironmental interactions, and the molecular transitions that underlie metastatic progression.
The application of SW480 cells in metastatic xenograft models enables the exploration of dynamic processes such as epithelial-to-mesenchymal transition, immune modulation, and extracellular matrix remodeling in a physiologically relevant context. By introducing SW480 cells into vascularized sites such as the spleen or tail vein, researchers can monitor their capacity to survive in circulation and establish secondary lesions in target organs, particularly the liver and lungs. These models are particularly useful for evaluating the efficacy of therapeutics aimed at interrupting metastatic spread and for identifying gene expression changes associated with the transition from localized to systemic disease. Although challenges remain in achieving consistent metastatic colonization, these models continue to advance our understanding of colorectal cancer metastasis and contribute to the development of strategies for therapeutic intervention and disease management.
TRIM67 Suppresses Notch Signaling and EMT in SW480 Cells
In the study published in BMC Cancer journal, Bond CE, et al. examine the tumor-suppressive function of PRDM5 in colorectal cancer, focusing on its ability to inhibit proliferation, invasion, and chemoresistance in SW480 cells. The authors demonstrate that PRDM5 expression is frequently downregulated in colorectal tumors and that its restoration in SW480 cells significantly reduces cell viability, colony formation, and invasive capacity. Apoptosis is notably increased, accompanied by the activation of caspase-3 and downregulation of the anti-apoptotic protein Bcl-2. Importantly, PRDM5 overexpression enhances the sensitivity of SW480 cells to 5-fluorouracil (5-FU), a frontline chemotherapeutic agent for colorectal cancer. In vivo, xenograft tumors formed from PRDM5-overexpressing SW480 cells display reduced size and elevated apoptotic activity, further supporting its tumor-inhibitory role. The findings support the hypothesis that PRDM5 serves as a suppressor of colorectal tumor progression and a modulator of chemotherapy response. The study employs a combination of molecular assays, functional tests, and xenograft modeling, providing robust evidence for the impact of PRDM5 on SW480 cellular behavior. While the data are compelling, the investigation is limited by its use of a single cell line and lacks mechanistic depth regarding PRDM5’s downstream signaling networks. Nonetheless, the results suggest that PRDM5 could function as both a predictive biomarker and therapeutic target in colorectal cancer, particularly in patients exhibiting poor response to 5-FU. Further research should explore PRDM5-mediated regulatory pathways and validate these findings across diverse cellular and clinical models.
Regulation of Smad2 and EMT by miR-145 in SW480 Cells
SW480 colorectal cancer cells display well-characterized changes in behavior and molecular phenotype in response to modulation of epithelial-mesenchymal transition (EMT) regulators. Upregulation of miR-145 in these cells leads to a significant reduction in proliferation and migratory capacity, which is accompanied by decreased expression of mesenchymal markers such as vimentin and N-cadherin. In parallel, the epithelial marker E-cadherin is upregulated, indicating a shift toward an epithelial phenotype and suppression of EMT. These changes reflect a reprogramming of cellular identity that aligns with reduced invasiveness and metastatic potential. At the molecular level, miR-145 directly downregulates Smad2 expression, a key mediator of the TGF-β signaling pathway, implicating this microRNA in the control of transcriptional networks that drive tumor progression. The relationship between miR-145 and tumor-suppressive activity in SW480 cells highlights the importance of microRNA-mediated regulation in colorectal cancer biology. By restoring miR-145 levels, it is possible to reverse the mesenchymal phenotype and impair cellular mechanisms associated with metastasis. These findings are supported by consistent alterations in gene and protein expression, as well as reduced performance in functional assays measuring cell migration and invasiveness. Techniques such as qPCR, western blotting, and transwell migration assays provide the necessary resolution to confirm these phenotypic effects. Although this insight is primarily drawn from in vitro models, the data suggest that miR-145 has significant therapeutic potential.
SW480 as a Model for Oncogenic Pathway Analysis
SW480 colorectal cancer cells provide a valuable system for examining the regulatory roles of specific oncogenes in tumor progression. KRAS, a proto-oncogene frequently mutated in colorectal cancer, exhibits constitutive activation in SW480 cells due to a missense mutation. This results in persistent signaling through downstream pathways such as MAPK and PI3K, contributing to increased proliferation, resistance to apoptosis, and enhanced invasive potential. Experimental silencing of KRAS in SW480 cells significantly reduces their growth rate, migration ability, and colony-forming efficiency, highlighting its essential role in maintaining the malignant phenotype. The expression of epithelial-mesenchymal transition (EMT) markers is also altered following KRAS knockdown, with reduced vimentin and increased E-cadherin, suggesting that KRAS contributes directly to the mesenchymal, motile phenotype characteristic of invasive colorectal tumors.
SW480 Colon Cancer CRC Subcutaneous, Orthotopic And Metastatic Xenograft Models ![]()
Basic study design
- Trypan blue exclusion is utilized to determine cell viability and cell count. After cell suspensions are diluted to the appropriate cell density for injection (10,000 cells/µL; injection volume = 100-200µL containing Matrigel), each immunocompromised mouse (10-12 w.o.) receives a single injection in the flank of the hind leg.
- Tumors are continually calipered (digital) tumor size averages are 75-125 mm3. Treatment cohorts are formed (randomization) the in-life study begins. All drug administration is performed according to the dosing schedule.
- Throughout the study tumors receive daily measurements, along with tri-weekly mouse weights recordings. As the predetermined tumor size is reached, animals are euthanized.
- Collected tissues are stored for downstream analysis (snap frozen, RNA-Later, 10% NBF or nucleic acid isolations). Tumor are weights and documented and digital images captured.
Get Instant Quote for
SW480 Xenograft Model
Altogen Labs provides an array of laboratory services using over 90 CDX and 30 PDX xenograft models. The dosing of the experimental compound of interest is initiated, for a staged study, when the mean tumor size reaches a specified volume (typically 75-125 mm3). In an unstaged study, the dosing of the test articles initiated 4-5 days after xenotransplantation. Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the SW480 xenograft model:
- SW480 Tumor Growth Delay (TGD; latency)
- SW480 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, continuous infusion, intraperitoneal, intratumoral, oral gavage, subcutaneous)
- SW480 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing dox or cyclophosphamide, at a dosages of 10-50 mg/kg administered QOD
