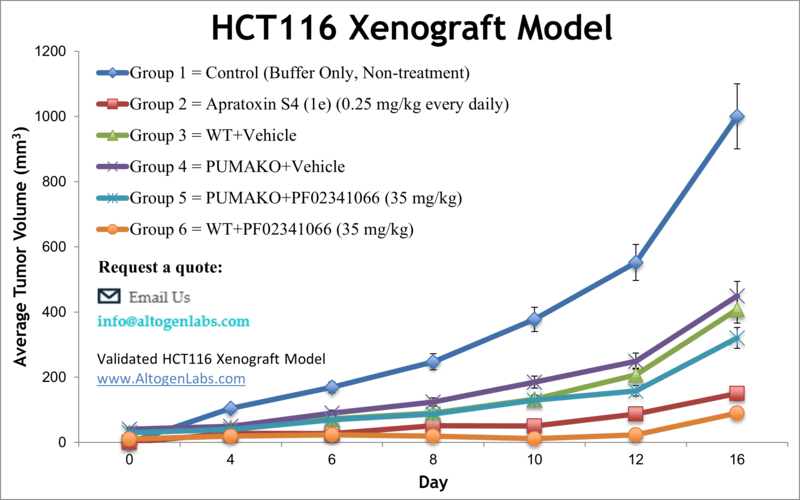
HCT116 xenograft model (subcutaneous and metastatic)
HCT116 is a commonly used human colorectal cancer cell line that was originally derived from the colon carcinoma of a 44-year-old male patient in 1979. The HCT116 cell line is widely used in cancer research as a model system to study various aspects of cancer biology, including cancer genetics, signaling pathways, drug discovery, and therapeutic interventions. HCT-116 cells are known for their ability to grow rapidly in culture and form tumors when injected into immunodeficient mice. They have been extensively characterized at the genetic and molecular levels, and the HCT116 genome has been fully sequenced. Novel therapeutic approaches are necessary for colon cancer patients to prevent disease relapse and progression. The incidence rates for colorectal cancer are on the rise in patients younger than age 50, even though it mostly affects older people, as per the Colon Cancer Alliance (CCA). Xenografting is the transplantation of tissues from one species to another, with xenotransplantation of human tumor cells into immunocompromised mice serving as a widely used technique in preclinical oncology research. Thoroughly designed animal models can contribute invaluable information for gaining further understanding of drug response in humans, enabling the discovery of new therapeutic agents. The HCT116 cell line was isolated from epithelial tumorigenic colon tissue of an adult male patient with colorectal carcinoma. Rajput et al. (2008) characterized HCT116 cells in an orthotopic model and reported primary tumor growth and the ability to form additional distant metastatic colonies in lung and liver, confirming HCT116 as an appropriate model system for examining the metastatic cascade. Guo et al. (2006) used HCT116 monolayers and xenografts to study sequential combination treatment of known chemotherapy drugs docetaxel, flavopiridol, and 5-fluorouracil (5-FU) and found that tumor growth and survival rate are dependent on order of drug administration; these results suggest that dosing and order of drug administration is critical in clinical combination therapy. The last example of a study using the HCT116 model is by Wang et al. (2015) which reported the effects of the bioactive polyhydroxysteroid Bufalin on colorectal cancer (CRC). The results reported significant tumor growth inhibition and increased survival with xenograft tumors; the mechanism of action is related to upregulation of apoptotic factors (Bad, Bax, PTEN and Caspase-3) as well as downregulation of p-AKT and Bcl-xL. These results help to indicate potential combination therapies in which Bufalin may be useful. The HCT116 cell line is used to create the CDX (Cell Line Derived Xenograft) HCT116 xenograft mouse model. Tumor growth inhibition of a therapeutic agent (such as docetaxel, 5-FU, flavopiridol) is ideal as a single agent or in combination using the HCT116 xenograft model. Researchers use the HCT116 xenograft model to study cancer in vivo, to evaluate the efficacy and toxicity of anticancer drugs, and to identify potential drug targets and molecular mechanisms of drug resistance. HCT116 cells have been used in a variety of research areas, including DNA damage response, cell cycle regulation, apoptosis, autophagy, and metastasis.
HCT-116 Orthotopic And Metastatic Xenograft Model: Download ![]()
Download Altogen Labs HCT116 Xenograft Model PowerPoint Presentation: ![]()
Subcutaneous Xenograft Modeling with HCT-116
Subcutaneous xenograft transplantation remains a foundational method in preclinical oncology for evaluating tumor growth kinetics, drug efficacy, and molecular responses in vivo. The HCT-116 colorectal carcinoma cell line, characterized by its microsatellite instability-high (MSI-H) status and oncogenic KRAS mutation, has been extensively employed in subcutaneous xenograft models due to its robust tumorigenicity and reproducible growth in immunodeficient mice. Early studies utilizing HCT-116 xenografts helped define dose-response relationships for standard chemotherapeutics such as 5-fluorouracil and irinotecan, while more recent investigations have employed this model to evaluate the activity of targeted agents, including MEK and PARP inhibitors. The subcutaneous implantation of HCT-116 cells into athymic BALB/C or NOD/SCID mice facilitates consistent tumor formation, with palpable masses typically forming within 7–10 days post-inoculation, enabling early intervention and longitudinal therapeutic studies. Although the subcutaneous model does not fully replicate the complex tumor-stroma interactions of orthotopic sites, it provides several critical advantages, including ease of monitoring tumor progression through caliper-based volume assessments and minimal procedural complexity. It is particularly useful for assessing tumor response in relation to genetic manipulations, such as stable RNAi-mediated knockdown or ectopic gene expression, both of which can be readily introduced into HCT-116 cells. Moreover, the HCT-116 xenograft model has contributed to investigations into immune modulation in MSI-H colorectal cancers, particularly in studies evaluating the impact of PD-L1 expression and immunotherapy resistance mechanisms. Ongoing efforts to enhance the physiological relevance of this model include co-injection with stromal fibroblasts or extracellular matrix components, enabling more accurate simulation of the tumor microenvironment. As such, subcutaneous transplantation of HCT-116 remains a vital and adaptable platform in colorectal cancer research, bridging molecular inquiry with translational application and supporting the development of personalized therapeutic strategies.
HCT-116 Xenografts in Metastatic Cancer Research
Metastatic xenograft transplantation provides a critical platform for modeling the progression of colorectal cancer from primary tumor formation to distant organ colonization. In this context, the HCT-116 cell line serves as a widely adopted model due to its inherent invasive properties and molecular profile characteristic of microsatellite instability-high (MSI-H) colorectal cancer. When introduced into anatomically relevant sites such as the spleen or cecum, HCT-116 cells demonstrate the ability to establish primary tumors and spontaneously disseminate to secondary organs, including the liver and lungs. These transplantation approaches more closely replicate the metastatic cascade observed in human disease, enabling the investigation of tumor cell-intrinsic and microenvironmental factors that drive metastatic competence. The application of metastatic HCT-116 models supports the evaluation of therapeutic strategies aimed at preventing or eliminating disseminated disease. These models enable detailed examination of the mechanisms underlying metastatic colonization, immune evasion, and drug resistance, offering insight into vulnerabilities that may not be apparent in non-metastatic settings. Additionally, the reproducibility and adaptability of these models make them suitable for genetic manipulation and longitudinal analysis, allowing researchers to track tumor evolution and treatment responses over time. By capturing the biological complexity of metastatic colorectal cancer, HCT-116 xenograft models serve as an indispensable resource for preclinical studies that seek to identify and validate effective therapeutic interventions for advanced-stage disease.
HCT-116 Orthotopic Xenografts in Tumor Progression Studies
Orthotopic xenograft transplantation has become an essential method in colorectal cancer research, offering enhanced biological relevance by recapitulating the native tumor microenvironment and tissue-specific interactions. In contrast to ectopic models, orthotopic transplantation of HCT-116 cells into the cecum or colon of immunodeficient mice enables the study of local tumor invasion, angiogenesis, and spontaneous metastasis in a manner that more closely mirrors clinical progression. The HCT-116 cell line, characterized by microsatellite instability and oncogenic KRAS mutation, reliably forms primary tumors when introduced into the colonic wall, exhibiting invasive phenotypes and the potential to disseminate to distant organs such as the liver and lungs. This localized implantation facilitates investigation into site-specific cues that govern tumor behavior, including interactions with colonic epithelium, extracellular matrix components, and regional vasculature. The orthotopic HCT-116 model serves as a valuable platform for assessing therapeutic responses within a physiologically relevant setting. By preserving anatomical and microenvironmental context, this approach enhances the predictive validity of preclinical drug studies, particularly those targeting invasion, immune modulation, and metastasis. Furthermore, orthotopic models support integration of advanced imaging technologies and longitudinal monitoring strategies, allowing for dynamic assessment of tumor growth, progression, and response to treatment. The incorporation of genetically modified HCT-116 derivatives, including stable knockdown or overexpression variants, adds further utility by enabling mechanistic dissection of gene function in vivo. As such, orthotopic transplantation of HCT-116 cells represents a robust and versatile system that bridges molecular insights with translational research objectives, advancing the understanding of colorectal cancer pathophysiology and informing therapeutic development.
HCT-116 Cell Growth and Migration Inhibited by Triterpenoid Extracts
Ethanol extracts from sporoderm-broken spores of Ganoderma lucidum (BSGLEE) exhibit potent inhibitory effects on HCT-116 colorectal cancer cells by targeting key processes involved in tumor progression. BSGLEE significantly reduces cell viability in a dose- and time-dependent manner, with progressively lower IC50 values observed over 24, 48, and 72 hours. This reduction in proliferation is linked to G0/G1 cell cycle arrest, as shown by flow cytometry, and accompanied by a decrease in the expression of cell cycle regulators including cyclin D1, cyclin E, CDK1, CDK2, and CDK4. At the same time, the tumor suppressor genes p16 and p21 are upregulated, suggesting a shift toward growth suppression. Apoptotic induction is confirmed through nuclear fragmentation visualized with Hoechst staining and increased levels of cleaved caspase-3 and PARP, along with elevated Bax and NAG-1 and reduced Bcl-2 expression. These findings collectively point to a coordinated activation of the intrinsic apoptotic pathway. In addition to impairing proliferation and inducing apoptosis, BSGLEE disrupts the migratory capacity of HCT-116 cells. Wound healing and Transwell migration assays demonstrate a significant, dose-dependent decline in cell motility. Molecular analysis reveals that BSGLEE downregulates motility-associated genes such as MMP-1, MMP-2, c-Met, and vimentin while enhancing the expression of E-cadherin, a key cell adhesion molecule. These results indicate a suppression of epithelial-mesenchymal transition features. In vivo, oral administration of BSGLEE delays tumor formation, reduces tumor volume and weight, and lowers proliferation markers such as Ki-67 in xenograft tumors. Apoptotic markers such as Bax are elevated in tumor sections, supporting the mechanistic observations in vitro. No signs of systemic toxicity or significant body weight loss are observed, and liver weights are partially restored in animals treated with higher doses. Together, these findings suggest that BSGLEE may act as a multi-targeted therapeutic agent that interferes with cell cycle progression, promotes apoptosis, and impedes metastatic behavior in colorectal cancer. Further research is warranted to explore its use in combination therapies and to clarify its immunomodulatory effects in immune-competent models.
Tumor Suppressor Role of SLC22A18 in Colorectal Cancer
This comprehensive investigation by Jung et al., published in Oncotarget journal, presents compelling evidence for SLC22A18 as a tumor suppressor in colorectal cancer, with HCT-116 cells serving as a key model. The researchers demonstrate that SLC22A18 expression is consistently downregulated in colorectal tumor tissues compared to matched normal tissues, a pattern corroborated by both clinical specimens and TCGA RNA-seq data. Functional assays reveal that ectopic expression of SLC22A18 significantly inhibits colony formation in HCT-116 cells and induces G2/M cell cycle arrest. Flow cytometry shows reduced S-phase entry and an increased G2/M population, accompanied by upregulation of p21 and downregulation of cyclin B1, Cdc25C, and phosphorylated Cdc2, aligning with tumor suppressor behavior. Notably, SLC22A18 expression is suppressed by oncogenic KRAS activity, and KRAS knockdown leads to marked upregulation of SLC22A18 in HCT-116 cells, suggesting a mutually inhibitory interaction. This is further supported by anchorage-independent growth assays in which SLC22A18 expression diminishes KRASG12D-induced transformation of NIH3T3 cells.
Nrf2 Activation Drives Biphasic Response in HCT-116 Cells
Sulforaphane, a dietary isothiocyanate known for activating the Nrf2 antioxidant pathway, exhibits a biphasic effect on colorectal cancer cells depending on p53 status. In HCT-116 cells with wild-type p53, low concentrations of sulforaphane stimulate proliferation, mitochondrial biogenesis, and antioxidant defense, while higher doses reduce cell viability. This proliferative effect is closely associated with increased nuclear Nrf2 and cytoplasmic HO-1 expression, an elevated Bcl-2 to Bax ratio, enhanced autophagy, and upregulation of mitochondrial biogenesis markers such as PGC1α. These cellular responses are notably absent or reversed in HCT-116 cells lacking p53, which instead display heightened apoptosis, reduced mitochondrial function, and impaired respiration upon sulforaphane treatment. The data suggest that the presence of p53 permits sulforaphane to activate a coordinated cytoprotective and pro-survival response, while its absence shifts the balance toward cell death. This p53-dependent divergence highlights the critical role of genetic context in modulating cellular responses to phytochemicals. The observed biphasic behavior underscores a risk associated with sulforaphane intake in colorectal cancers retaining wild-type p53, where it may inadvertently support tumor growth. Experimental models further confirm that sulforaphane promotes proliferation in p53-competent xenografts but has minimal effect or promotes apoptosis in p53-deficient tumors. These findings challenge the broad categorization of sulforaphane as universally anticancer and call for more nuanced use of Nrf2-activating compounds.
Basic study design
- Flasks are maintained at exponential growth phase prior to collection.
- The cells are trypsinized and cell count/viability is determined by trypan blue (min req 98% viability). Cell suspensions are then diluted to the required concentration for inoculation.
- One million cells of the Matrigel + HCT116 cell suspension (vol of 100 µL) is injected s.c. into the hind leg of all mice (NOD/SCID or athymic BALB/C, 10-12 w.o.).
- The injection sites are observed until tumors are palpable. Digital calipers are used to measure tumors. Initial grouping starts when tumors reach sizes of 50-150 mm3.
- Post-sorting into the required treatment groups, the test compound of interest is injected following the treatment schedule.
- Tumor measurements are taken daily and mouse weights are recorded (3 times weekly).
- As the study tumor size reaches the predetermined tumor size limit, animals are euthanized.
- As decided for the end of the experiment, necropsy and tissue collection is performed. Tumors are removed, weighed and documented (digital imaging).
- Tissues and tumors are collected for further analysis by a standard gross necropsy.
Metastatic Model
CDX models are mouse xenografts used in pre-clinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics).
To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-luc Xenograft Model
An example of Altogen Labs utilizing a luciferase expressing cell line to monitor orthotopic tumor growth is exhibited below. The same ideology of tumor observation is incorporated in metastatic tumor models.
Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
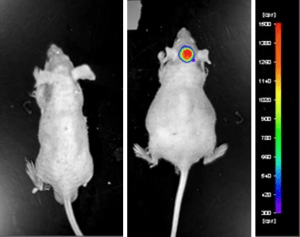
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model fluorescence was analyzed 10 minutes after intraperitoneal luciferin injection.
View full details of the case study by clicking here.
Get Instant Quote for
HCT116 Xenograft Model
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
HCT-116 Orthotopic And Metastatic Xenograft Model: Download ![]()
Following options are available for the HCT116 xenograft model:
- HCT116 Tumor Growth Delay (TGD; latency)
- HCT116 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, continuous infusion, intraperitoneal, intratumoral, oral gavage)
- HCT116 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Imaging studies: Fluorescence-based whole body imaging, MRI
