CT26 syngeneic murine model
Colorectal cancer (CRC) remains a leading cause of cancer-related morbidity and mortality worldwide, and despite notable therapeutic advances, resistance to standard-of-care combination chemotherapy remains a significant clinical challenge. The development of novel treatment strategies, particularly those that engage the immune system or target the tumor microenvironment, is essential to improving outcomes. The CT26 cell line, derived from an undifferentiated colon carcinoma induced by N-nitroso-N-methylurethane (NNMU) in BALB/c mice, serves as a widely used preclinical model for immunocompetent syngeneic tumor studies. Unlike xenograft models utilizing human tumor cells implanted into immunodeficient hosts, syngeneic models such as CT26 preserve intact immune functionality and are thus particularly valuable for evaluating immuno-oncology therapies.
The CT26 model has been instrumental in advancing understanding of tumor immunogenicity and evaluating immune-based therapeutics. As a fully murine colon carcinoma cell line implanted into genetically matched BALB/c mice, the CT26 syngeneic model enables detailed analysis of immune cell infiltration, cytokine responses, and immune checkpoint modulation in a physiologically relevant setting. Studies using this model have demonstrated the potential of immunotherapies to stimulate tumor-infiltrating cytotoxic T lymphocytes, enhance the presence of antigen-presenting dendritic cells, and reduce immunosuppressive myeloid-derived suppressor cell (MDSC) populations. The model has also been employed to evaluate novel delivery platforms, such as adenoviral vectors encoding immunostimulatory genes, and combination treatments involving conventional agents like 5-fluorouracil, oxaliplatin, and autophagy inhibitors. Moreover, antibody-cytokine fusion strategies, including HER2-targeted endostatin fusion proteins, have shown promise in enhancing anti-angiogenic effects and overcoming pharmacokinetic limitations. Collectively, the CT26 model remains a cornerstone of preclinical CRC research, offering a robust platform for the discovery and optimization of next-generation immunotherapies and targeted treatments.
CT26 Allograft Model: Download ![]()
Download Altogen Labs CT26 Syngeneic Mouse Model PowerPoint Presentation: ![]()
CT26 Cell Line
The CT26 murine colon carcinoma cell line, originally derived from a chemically induced tumor in BALB/c mice, is extensively utilized in preclinical cancer research as a syngeneic model of microsatellite stable colorectal cancer. It is characterized by a KrasG12D mutation and exhibits high immunogenicity, with a tumor microenvironment rich in cytotoxic T lymphocytes, macrophages, and myeloid-derived suppressor cells. CT26 has become a cornerstone in evaluating the efficacy of immunotherapies, including checkpoint inhibitors, adoptive T cell transfer, and mRNA-based vaccines. Studies have demonstrated partial sensitivity of CT26 tumors to PD-1 blockade, with enhanced responses observed when used in combination with agents targeting innate immunity or costimulatory pathways. The model has also supported the development of nanoparticle-mediated drug delivery systems and oncolytic virotherapies. However, its artificial origin and murine-specific oncogenic profile limit its translational applicability, particularly regarding mechanisms of immune evasion and tumor-stroma interactions observed in human colorectal cancers. Furthermore, discrepancies in immunotherapy responsiveness across experimental conditions underscore the need for deeper characterization of the tumor immune landscape within this model.
Subcutaneous Transplantation of CT26 for Immune Response Assessment
Subcutaneous allograft transplantation using the CT26 murine colon carcinoma model represents a widely utilized and well-characterized approach in preclinical cancer research. This model offers distinct advantages, including consistent tumor engraftment, ease of tumor measurement, and compatibility with immunocompetent BALB/c mice, allowing researchers to evaluate immune-mediated responses within a physiologically relevant context. Unlike allografts established in immunodeficient hosts, the CT26 model preserves the functional integrity of the immune system, enabling the assessment of immunotherapies such as checkpoint inhibitors, cytokine-based treatments, and oncolytic agents. Studies such as those by Lechner et al. have highlighted the value of this model in characterizing tumor-infiltrating lymphocytes and identifying immune biomarkers predictive of therapeutic response.
While orthotopic and metastatic models offer site-specific insights, subcutaneous transplantation remains essential for standardized efficacy studies and mechanistic investigations. The accessibility of subcutaneous tumors permits longitudinal monitoring and repeated sampling, facilitating downstream analyses including flow cytometry, histology, and gene expression profiling. Research by Zhao et al. has shown that despite anatomical differences, subcutaneous CT26 tumors can support robust immune activation, particularly when combined with immunomodulatory agents. Although limitations exist regarding the lack of organ-specific microenvironments, the CT26 subcutaneous model remains a critical platform for early-stage therapeutic evaluation and continues to inform the rational development of immuno-oncology strategies.
Orthotopic CT26 Models for Colorectal Cancer Research
Orthotopic allograft transplantation provides a biologically relevant platform for modeling tumor growth, immune interactions, and therapeutic response within the anatomical site of origin. In colorectal cancer research, the CT26 cell line can be orthotopically implanted into the cecal wall of syngeneic BALB/c mice, creating a tumor microenvironment that closely mimics the structural and immunological features of primary colon tumors. Unlike subcutaneous models, orthotopic transplantation supports the development of tissue-specific vasculature and facilitates interactions with regional immune cells, fibroblasts, and extracellular matrix components. This approach is particularly valuable for evaluating tumor progression, local invasion, and the mechanisms driving site-specific metastasis, which are often not recapitulated in ectopic models.
The CT26 orthotopic model offers a unique opportunity to investigate immune-based therapies within a physiologically relevant setting. Its compatibility with immunocompetent hosts enables rigorous assessment of tumor-immune dynamics, including T cell infiltration, antigen presentation, and cytokine signaling within the gastrointestinal microenvironment. This model is well-suited for studying the influence of the tumor niche on therapeutic outcomes and for identifying factors that contribute to immune evasion and treatment resistance.
Metastatic Progression and Immune Response in CT26 Models
Metastatic allograft transplantation is critical in preclinical oncology, providing a platform to investigate the biological processes underpinning cancer dissemination and to evaluate therapies aimed at preventing or treating metastatic disease. The CT26 murine colon carcinoma model, widely used in colorectal cancer research, is particularly well-suited for generating metastatic allograft systems due to its aggressive growth kinetics and immunogenic profile. When introduced into syngeneic BALB/c mice, CT26 cells can give rise to secondary tumor sites through both spontaneous and experimental routes of metastasis, enabling researchers to study organ-specific colonization patterns and immune responses in an immunocompetent setting.
Metastatic CT26 models are typically established through either orthotopic implantation followed by natural dissemination or intravenous injection to target specific metastatic sites such as the lungs. These approaches allow for reproducible assessments of metastatic potential and therapeutic response. Moreover, the use of luciferase-expressing CT26 variants facilitates real-time, non-invasive monitoring of tumor burden and metastatic progression. This model system supports detailed immunological and molecular characterization of metastasis, including the analysis of immune suppressive pathways, cytokine signaling, and stromal interactions. By capturing key features of the metastatic cascade within a syngeneic environment, CT26 metastatic models offer a valuable preclinical framework for evaluating novel interventions and improving therapeutic strategies for advanced-stage colorectal cancer.
Assessing Therapeutic Efficacy and Immune Interaction in CT26 Tumors
The CT26 murine model of colorectal cancer provides a valuable system for evaluating therapies that target oncogenic K-Ras mutations, especially the G12D variant, which is common in gastrointestinal tumors. A bicyclic peptide inhibitor developed for K-Ras(G12D) was found to suppress CT26 cell proliferation and colony formation in a dose-dependent manner, accompanied by reduced phosphorylation of ERK, a key signaling mediator downstream of Ras. Although the peptide requires micromolar concentrations to achieve biological effects, likely due to limited cell membrane permeability, it induces modest apoptosis and primarily restricts tumor growth through cytostatic mechanisms. In vivo, the compound significantly reduces tumor volume in CT26 allografts without causing weight loss or pathological changes in the liver or kidneys, indicating a favorable safety profile for further preclinical development.
However, when combined with an immune checkpoint inhibitor, no additive or synergistic effect was observed. Analysis of CT26 tumor samples showed no significant differences in CD8+ T cell infiltration or PD-L1 expression among treatment groups. Pharmacokinetic profiling revealed a short plasma half-life, strong plasma protein binding, and notable accumulation within blood cells, all of which may limit systemic availability and interfere with immunotherapeutic interactions. These findings underscore the importance of considering drug distribution, immune modulation, and delivery mechanisms when designing combination therapies. Further investigation using metastatic or orthotopic CT26 models, along with improved formulations or delivery platforms, will be essential to realize the full therapeutic potential of K-Ras(G12D)-targeting agents.
Immune Context Shapes Oncogenic Signaling in CT26 Tumors
The CT26 murine colon carcinoma model is valuable for studying the effects of immune context on tumor evolution, particularly the reprogramming of oncogenic signaling and immune recognition. In immunocompetent hosts, CT26 tumors typically grow rapidly, showing low antigenicity and moderate immune infiltration. However, when introduced into immunocompromised hosts such as NOD.SCID mice, early tumor growth slows markedly, despite the concurrent silencing of key tumor suppressors including PTEN and RBL1. Proteomic analysis of CT26 tumors revealed a significant reprogramming of intracellular signaling pathways in the absence of immune pressure, notably involving the PI3K/AKT/mTOR and TP53 networks. This shift appears to facilitate survival in a stressed tumor microenvironment while simultaneously enhancing genomic instability. As tumors progress in this immunodeficient context, they begin to express elevated levels of tumor-associated antigens and become increasingly immunogenic.
Re-inoculation of these CT26 tumors into immunocompetent BALB/c mice further demonstrated their heightened immunogenic profile. Approximately 70 percent of the tumors originally grown in NOD.SCID mice regressed upon transfer, in contrast to near-complete progression observed with CT26 tumors derived from BALB/c hosts. This regression was accompanied by a robust infiltration of antigen-presenting cells and CD3+ T lymphocytes, indicating effective activation of adaptive immunity. The findings suggest that the absence of immune editing in immunodeficient hosts permits the accumulation of immunogenic tumor subclones with preserved or elevated antigen expression. This mechanism may underlie the paradoxical observation that tumors from immune-deficient environments can become more immunogenic over time. Furthermore, the data imply that stress-induced signaling alterations, including endoplasmic reticulum stress and deregulation of protein processing pathways, may act as co-factors in antigen emergence. These insights position CT26 as a powerful model for understanding immune-tumor coevolution, tumor antigenicity, and the design of strategies to enhance the efficacy of cancer immunotherapies through controlled modulation of the tumor microenvironment.
Advancing Immunotherapy with Syngeneic Mouse Models
Syngeneic mouse models represent a foundational platform in cancer research, particularly for elucidating interactions between tumor cells and the host immune system. These models are established by transplanting tumor cells into genetically identical, immunocompetent mice, thereby preserving the integrity of immune responses that would be absent in immunodeficient systems. Their capacity to replicate native immune-tumor dynamics makes them especially well-suited for preclinical evaluation of immunotherapies. Syngeneic models enable detailed investigation into immune evasion mechanisms, immune cell infiltration, and the functional consequences of therapeutic interventions on both innate and adaptive immune responses. Moreover, they provide a robust framework for studying metastatic dissemination, tumor microenvironmental modulation, and immune-mediated tumor clearance. By maintaining a fully functional immune system, these models yield insights that are highly relevant to the clinical development of immunomodulatory cancer therapies.
Basic study design
- CT26 cells used for injection are continually maintained under exponential growth.
- The cells are prepared for injection via trypsinization. Viable cell counts are determined using a trypan blue exclusion (98% cell viability required). The cell suspension concentration is adjusted to the appropriate density.
- Each mouse (BALB/C 10-12 weeks old) receives a single subcutaneous injection into the flank of one hind leg containing 0.1-1 mil cells. The volume of the injection is 100 µL of the Matrigel + CT26 cell suspension.
- All injection sites are palpated up to three times a week until tumors are established. Tumors are measured using a digital caliper until an average size of 50-75 mm3 is reached.
- Animals are then randomized into treatment cohorts and the compound of interest is administered according to the treatment schedule.
- Tumors are measured daily, and mouse weights are recorded up to 3 times a week.
- Animals are euthanized as tumor size nears 2,000 cu millimeters, or predetermined study size limit.
- Tumor collections are performed as defined for termination of experiment.
- All tumors are excised and weighed, then documented with a digital image.
- Gross necropsies are performed and the tissues of interest are collected for downstream analysis.
- Tumors/tissues can be stabilized in RNA-Later, snap frozen and prepared for histology.
Get Instant Quote for
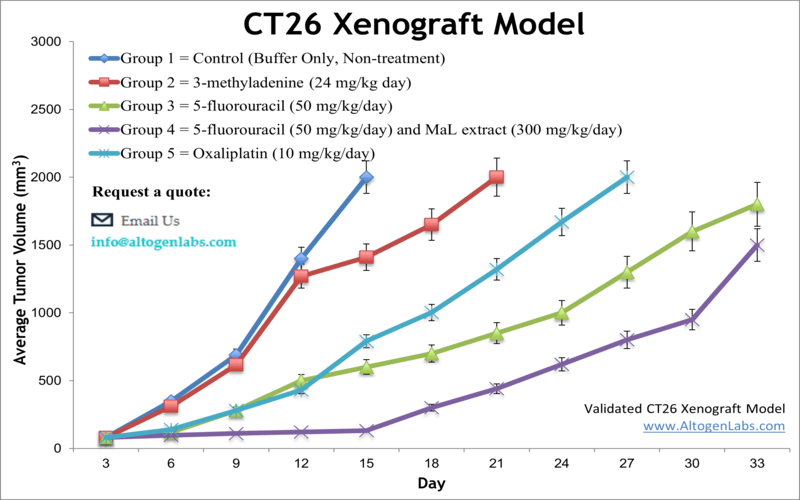
CT26 Xenograft Model
Altogen Labs provides an array of laboratory services using over 90 standard Cell Line Derived Xenograft (CDX) models and over 30 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (qRT-PCR) and protein expression analysis using the WES system (ProteinSimple). Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
CT26 Syngeneic Allograft Model: Download ![]()
The following options are available for the CT26 syngeneic mouse model:
- CT26 Tumor Growth Delay (TGD; latency)
- CT26 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- CT26 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection for metastasis studies)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
