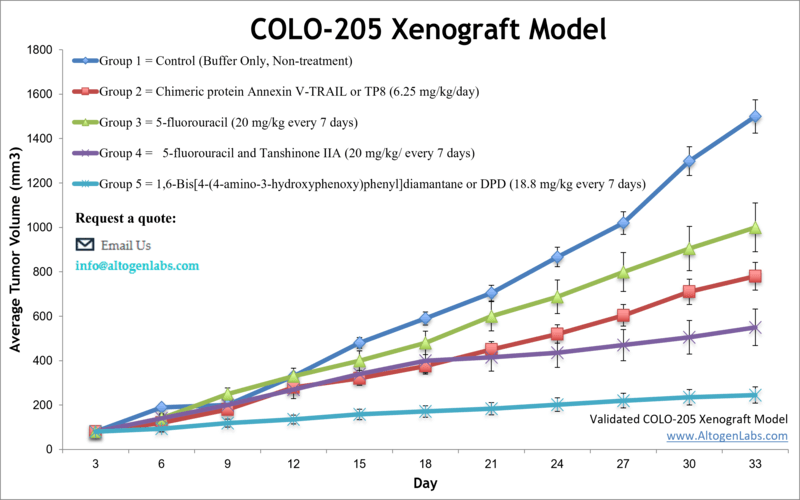
COLO-205 xenograft model
Colorectal carcinoma (CRC) is the second common cause of cancer-related deaths, mostly occurring in people over 50. Preclinical studies of CRC can help researchers to investigate its correlation with immune response and develop new treatment approaches. The COLO-205 epithelial cell line was isolated by Dr. Semple in 1975 from a 70-year-old Caucasian male patient with colorectal adenocarcinoma after the treatment with 5-fluorouracil (5-FU) for roughly five weeks. The COLO-205 cell line originates from a Dukes’ type D tumor and expresses a 36,000-dalton cell surface glycoprotein. These cells are negative for expression of CSAp and keratin positive. The COLO-205 cell line is used for researching the SV-40 monkey virus and serves a suitable host for studying colon cancer. Rodent xenograft models share a vast array of characteristics with humans and act as indispensable tools in the development of newer chemotherapeutic drugs. The COLO-205 model has been used in many studies including by Cheng et al. (2010) which investigated potential combination therapy of 5-fluorouracil with traditional Chinese medicine prescriptions; results showed inhibition of tumor growth and microtubule-associated protein light chain 3 (MAP-LC3-II) in xenograft models, suggesting therapeutic potential for Sann-Joong-Kuey-Jian-Tang (SJKJT). A 2009 study by Oliver et al. used a COLO-205 model to evaluate the monoclonal antibody TRA-8, which binds to the DR5 death receptor and activates tumor necrosis factor-related apoptosis inducing ligand (TRAIL/Apo2L). They found that TRA-8 was most effecting in producing in vitro toxicity and in vivo tumor regression on DR5-expressing cancers when used in combination with CPT-11, a compound commonly used to treat metastatic colon cancers. Lastly. in 2011, Yalcin et al. published a study comparing the commonly used colon cancer chemotherapy treatment cetuximab (a monoclonal antibody against epidermal growth factor receptor) to tetraiodothryacetic acid (tetrac), a deaminated analog of L-thyroxine that binds to alphavbeta3 integrin thyroid hormone receptor and potentially modifies EGFR expression and activity. Results showed that the tetrac nanoparticulate ((poly-[lactate-co-glycolic acid])-tetrac (tetrac-NP)) equally suppress COLO-205 xenograft growth as compared to cetuximab. The Colo205 xenografts have been used to study a wide range of topics in cancer biology, including the role of specific genes and signaling pathways in cancer development, the effects of chemotherapy and radiation therapy on cancer cells, and the potential of novel therapeutics for treating colon cancer. COLO205 cells are tumorigenic in nude mice and used to create the cell-line derived COLO-205 xenograft mouse model. The COLO-205 colon tumor model is an established model to study the in vivo efficacy of 5-FU or bevacizumab.
Validated COLO-205 Xenograft Model: Subcutaneous And Orthotopic Tumor Model: ![]()
Altogen Labs COLO205 Xenograft Model PowerPoint Presentation: ![]()
COLO205 Cell Line
The COLO205 cell line, established from the ascitic fluid of a patient with colorectal adenocarcinoma, serves as a widely utilized in vitro model for investigating the molecular and pharmacological characteristics of colorectal cancer. This epithelial cell line exhibits a microsatellite-stable genotype and harbors a homozygous BRAF^V600E mutation, while remaining wild-type for KRAS and TP53, thereby representing a genetically defined subset of colorectal tumors with distinct clinical behavior. COLO205 has been extensively employed in studies evaluating MAPK pathway inhibitors, particularly BRAF-targeted therapies, which demonstrate initial efficacy but are frequently undermined by adaptive resistance through EGFR-mediated signaling feedback. Combination regimens incorporating BRAF and EGFR inhibitors have shown synergistic cytotoxicity, although resistance mechanisms such as alternative receptor tyrosine kinase activation and PI3K/AKT pathway engagement continue to pose therapeutic challenges. Additionally, COLO205 exhibits a pronounced apoptotic response to targeted agents, rendering it a valuable model for dissecting programmed cell death mechanisms.

Targeting Oncogenic Signaling in COLO205 Through FRα-Mediated TP53 Activation
COLO205, a colorectal cancer cell line expressing wild-type TP53 and folate receptor alpha (FRα), provides a valuable model for investigating nutrient-regulated oncogenic signaling. Exposure to folic acid (FA) significantly reduces COLO205 proliferation through a defined signaling cascade involving FRα, c-SRC, ERK1/2, and NFκB. This pathway culminates in the upregulation of TP53 and its downstream targets CDKN1A (p21) and CDKN1B (p27), resulting in G0/G1 cell cycle arrest. The anti-proliferative effect was confirmed both in vitro and in vivo, where FA treatment led to substantial tumor regression and increased lifespan in xenograft-bearing mice. Inhibition of any pathway component or TP53 itself abolished the effects, confirming the pathway’s specificity. Chromatin immunoprecipitation further supported NFκB binding to the TP53 promoter, establishing transcriptional activation as a central mechanism. Additionally, angiogenesis was impaired, as indicated by reduced von Willebrand factor expression in treated tumors. The signaling cascade activated by FA was shown to be tightly regulated and time-dependent, with a transient increase in TP53 and its targets, likely due to feedback through MDM2-mediated degradation. Despite this narrow activation window, longer FA exposure restored elevated protein levels, supporting its sustained regulatory role. The COLO205 model’s expression of FRα and responsiveness to FA make it an ideal system for studying folate-targeted therapies. Methodologically, the research was well controlled, using pharmacologic inhibitors, gene knockdown, and multiple validation assays. Future studies should investigate FA’s effects in immune-competent systems, assess therapeutic synergy with existing chemotherapies, and further explore the regulation of CDKN1B by TP53. These findings support COLO205’s relevance in modeling FA-responsive signaling and underscore the broader therapeutic potential of targeting folate-regulated pathways in colorectal cancer.
Subcutaneous Xenografts in Colorectal Cancer Research
Subcutaneous xenograft transplantation using the COLO205 cell line is a widely employed preclinical approach for modeling human colorectal cancer, offering a reproducible and accessible system for evaluating tumor growth and therapeutic response. Derived from a patient with advanced colorectal adenocarcinoma, COLO205 cells harbor the BRAF^V600E mutation and have been instrumental in studies investigating targeted inhibition of the MAPK pathway. Typically injected into the flank of immunocompromised BALB/c nude mice in a Matrigel suspension, COLO205 cells form measurable tumors within two weeks, enabling longitudinal assessment of drug efficacy. This model has demonstrated sensitivity to BRAF and EGFR inhibitors, particularly in combination, and has been used to characterize mechanisms of acquired resistance involving PI3K/AKT and MET signaling. Recent investigations have leveraged COLO205 xenografts to explore gene expression dynamics, apoptosis regulation, and metabolic adaptation under therapeutic stress, revealing exploitable vulnerabilities. Although limited by its lack of immune components and non-orthotopic tumor growth, the COLO205 subcutaneous model remains a critical platform for translational colorectal cancer research, supporting the development and refinement of targeted treatment strategies.
In vivo Characterization of COLO205 in Orthotopic Transplantation
Orthotopic xenograft transplantation using COLO205 cells provides a biologically relevant model for studying colorectal cancer by replicating the tumor’s native anatomical environment and local microenvironmental interactions. This approach surpasses the limitations of subcutaneous models by enabling investigation of tumor progression, invasion, and early metastatic events within the colon. Studies employing COLO205 cells engineered to express luciferase and GFP have demonstrated successful engraftment into the submucosa of the mouse cecum, allowing for non-invasive monitoring of tumor growth and regional lymphatic spread. While distant metastasis to organs such as the liver and lungs is infrequent in this model, the localized tumor development closely mimics early-stage clinical disease. Orthotopic models using COLO205 have proven valuable in assessing therapeutic responses under more clinically relevant conditions, particularly in relation to drug penetration, stromal interactions, and local tissue architecture. Although the use of immunodeficient hosts restricts the study of immune-based therapies, this model facilitates in-depth analysis of tumor-intrinsic features, epithelial-mesenchymal transition, and resistance mechanisms. As such, COLO205 orthotopic xenografts offer a critical platform for advancing translational research and preclinical evaluation of novel treatments in colorectal cancer.
Targeting VEGF-A and Ang-2 Suppresses Tumor Growth and Metastasis
The COLO205 colorectal cancer xenograft model provides a robust platform for evaluating angiogenesis-targeting therapies and has demonstrated high sensitivity to dual inhibition of vascular endothelial growth factor A (VEGF-A) and angiopoietin-2 (Ang-2). A tetravalent bispecific antibody targeting both ligands achieved an 87 percent reduction in tumor growth, outperforming VEGF-A inhibition alone, which resulted in 66 percent tumor growth inhibition, and Ang-2 inhibition alone, which produced a 47 percent reduction. This combined approach remained effective even after the development of resistance to VEGF-targeted therapy, leading to a 60 percent decrease in tumor volume compared to continued VEGF-A monotherapy. The bispecific antibody also significantly suppressed lung metastasis by 66 percent. Histological examination revealed extensive tumor cell death and near-complete inhibition of microvessel formation in treated tumors, confirming its anti-angiogenic and anti-metastatic effects. These findings highlight the potential of COLO205 xenografts to model therapeutic resistance and validate the strategy of targeting multiple angiogenic pathways to enhance treatment efficacy. Patterns in the data consistently show superior outcomes with dual-targeting approaches across key measures such as tumor regression, metastasis suppression, and vascular density reduction. The COLO205 model is particularly suited for this type of evaluation due to its reproducibility, human VEGF dependency, and capacity to reflect adaptive mechanisms such as Ang-2 upregulation following VEGF-A inhibition. These characteristics provide insights into how tumors may circumvent single-pathway blockade and underscore the importance of simultaneous pathway targeting. Methodologically, the model supports reliable measurements of tumor volume, histological changes, and metastatic burden. However, the use of immunodeficient hosts limits evaluation of immune-related effects, and lack of cross-reactivity with host VEGF may lead to an underestimation of effects on the tumor microenvironment.
Dual Treatment Suppresses Drug Resistance and Metastasis in COLO205
The COLO205 colorectal cancer cell line serves as an effective in vivo platform for evaluating synergistic drug responses, particularly in the context of adjuvant chemotherapy. Treatment with a combination of 5-fluorouracil (5-FU) and Tanshinone IIA (Tan-IIA), a diterpene quinone derived from Salvia miltiorrhiza, resulted in substantial tumor volume reduction compared to 5-FU monotherapy or vehicle controls. Over a four-week treatment period in SCID mouse xenograft models, the combined therapy reduced average tumor volumes by over 50 percent relative to 5-FU alone, with no significant adverse effects on animal weight. Western blot analyses revealed that the combination therapy led to downregulation of several key proteins associated with drug resistance, angiogenesis, autophagy, and metastasis. These include P-glycoprotein (P-gp), VEGF, LC3-II, MMP-7, and NF-κB p65, all of which were expressed at significantly lower levels in tumors treated with 5-FU plus Tan-IIA than in those treated with 5-FU alone. The expression patterns of these biomarkers suggest a coordinated suppression of pathways that normally promote therapeutic resistance and tumor progression. P-gp downregulation implies reduced efflux of chemotherapeutic agents, enhancing intracellular 5-FU efficacy. The observed decrease in LC3-II, a marker of autophagy, is particularly significant, as autophagy can allow tumor cells to survive cytotoxic stress. Inhibition of VEGF and MMP-7 indicates suppression of angiogenesis and metastatic potential, while the reduction of NF-κB p65 suggests diminished inflammatory and survival signaling. These findings align with prior evidence that inhibition of NF-κB enhances 5-FU sensitivity. The methodology employed in this study, including rigorous protein quantification and appropriate controls, supports the reliability of the results. However, limitations include the use of immunodeficient mice and a narrow treatment window, which may not fully reflect clinical complexity. Still, COLO205 proves to be a powerful model for evaluating combination therapies, especially those targeting drug resistance and metastatic signaling. Further studies should explore the long-term durability of these effects, integration with immune-competent systems, and potential for clinical translation in human colorectal cancer treatment.
Basic study design
- COLO-205 cells for injection are maintained under aseptic conditions and at exponential growth phase.
- The COLO-205 cells are trypsinized and cell viability determined using a trypan blue exclusion assay (minimum 98% cell viability). The cell suspension is adjusted to appropriate density prior to inoculation.
- Each mouse (athymic BALB/c (nu/nu), 10 weeks old) receives a single subcutaneous injection into the flank of a hind leg containing 1 x 10^6 cells in 100 µL of the Matrigel+COLO-205 cell suspension.
- The injection sites are examined three times weekly until tumors are established. Tumors are measured utilizing digital calipers, with optimal starting tumor volumes at an average size of 50-150 mm3.
- Animals are randomized into determined treatment cohorts, with administration of the compound of interest injected according to the treatment schedule.
- Daily, tumors are measured and weights of mice are recorded up to 3 times weekly.
- Animals are euthanized as tumor size approaches either 2,000 sq millimeters or the predetermined tumor size limit.
- A necropsy is performed and tissues collected defined in the termination of experiment.
- Tumors are excised from the animal, weighed and documented using digital imaging.
- A standard gross necropsy is performed and tissues are collected according to customer request for downstream analysis.
- Tumors and/or tissues can be frozen in LN2, prepared for histology or stabilized in RNAlater for gene expression analysis.
- Animals were housed in a pathogen-free animal facility in accordance with the Guide for Care and Use of Laboratory Animals, along with the regulations of the Institutional Animal Care and Use Committee (IACUC).
Get Instant Quote for
COLO-205 Xenograft Model
Xenograft animal models serve as a foundational platform for evaluating the antitumor efficacy of investigational compounds against specific cancer types. These models involve the engraftment of human tumor cells into immunodeficient murine or rat hosts via subcutaneous or orthotopic injection, enabling the study of tumor growth kinetics and drug response in a biologically relevant in vivo environment. All currently approved oncology therapeutics have undergone rigorous preclinical validation using such xenograft systems. The design and execution of xenograft studies involve multiple critical parameters, including the selection of an appropriate host species, tumorigenic cell line, route and frequency of administration, and comprehensive endpoints such as tumor volume measurements, histopathological assessments, and quantification of molecular biomarkers at the transcript (mRNA) and protein levels. Altogen Labs offers a broad portfolio of preclinical research services utilizing over 90 established cell line-derived xenograft (CDX) models and many patient-derived xenograft (PDX) models. For researchers investigating tumor biology or therapeutic mechanisms of action, Altogen Labs supports the generation of custom cell lines engineered for stable protein overexpression or RNA interference-mediated gene silencing. Molecular characterization services include high-sensitivity quantification of gene expression via reverse transcription PCR (RT-PCR) and protein analysis using the capillary-based WES system (ProteinSimple), enabling precise evaluation of target engagement and downstream pathway modulation. All in vivo studies at Altogen Labs are conducted in accordance with Institutional Animal Care and Use Committee (IACUC) regulations and adhere to Good Laboratory Practice (GLP) guidelines. Mice are acclimated to the vivarium environment and randomized based on body weight prior to study initiation. Routine monitoring includes daily evaluation for tumor emergence, animal health status, and behavioral indicators. A comprehensive study report is provided upon completion, encompassing detailed methodology, statistical analysis, raw datasets, and interpretation of results. Optional services include tissue collection, histological processing, nucleic acid and protein isolation, and gene expression profiling to support advanced translational research objectives.
Validated COLO-205 Xenograft Model: Subcutaneous And Orthotopic Tumor Model: ![]()
Following options are available for the COLO-205 xenograft model:
- COLO-205 Tumor Growth Delay (TGD; latency)
- COLO-205 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- COLO-205 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
