Breast cancer remains one of the most prevalent and deadly malignancies worldwide, with diverse subtypes that vary in aggressiveness and treatment response. To better understand tumor biology and develop effective therapies, researchers utilize xenograft models, where human breast cancer cells or patient-derived tumor fragments are implanted into immunodeficient mice. These models closely mimic the tumor microenvironment, allowing for the study of cancer progression, metastasis, and therapeutic resistance in a controlled setting. Xenografts are particularly valuable in preclinical drug testing, enabling researchers to evaluate the efficacy of novel treatments before clinical trials. Patient-derived xenografts (PDXs) retain the genetic and histological characteristics of the original tumors, making them highly predictive of patient responses. Additionally, cell line-derived xenografts (CDXs), which involve the implantation of established cancer cell lines, offer a reproducible and cost-effective alternative for screening therapies and studying cancer biology in a consistent model system.
SKBR3 Subcutaneous And Orthotopic Model: Download ![]()
SK-BR-3 Cell Line
The SK-BR-3 cell line is a widely used human breast cancer cell line that originates from a pleural effusion of a female patient with metastatic breast carcinoma. It is characterized as a HER2-positive (human epidermal growth factor receptor 2) cell line, meaning it overexpresses the HER2 receptor, which is associated with aggressive tumor growth and poor prognosis in breast cancer. SK-BR-3 cells exhibit a moderate to high level of HER2 amplification, making them a useful model for studying HER2-targeted therapies, such as trastuzumab (Herceptin). These cells are typically epithelial-like in morphology and are known to form monolayers when cultured in vitro. SK-BR-3 cells are estrogen receptor-negative, progesterone receptor-negative, and do not express significant amounts of androgen receptors, making them a representative model for triple-negative breast cancer research and are often used to investigate cell signaling pathways, drug resistance, and mechanisms of metastasis. The cell line is frequently used in both basic research and preclinical drug development, particularly for testing novel therapies targeting HER2 signaling. Due to their tumorigenic properties, SK-BR-3 cells are also utilized to assess the efficacy of chemotherapy and immunotherapy agents in vitro and in xenograft models.
Altogen Labs Validated SK-BR-3 Xenograft Model
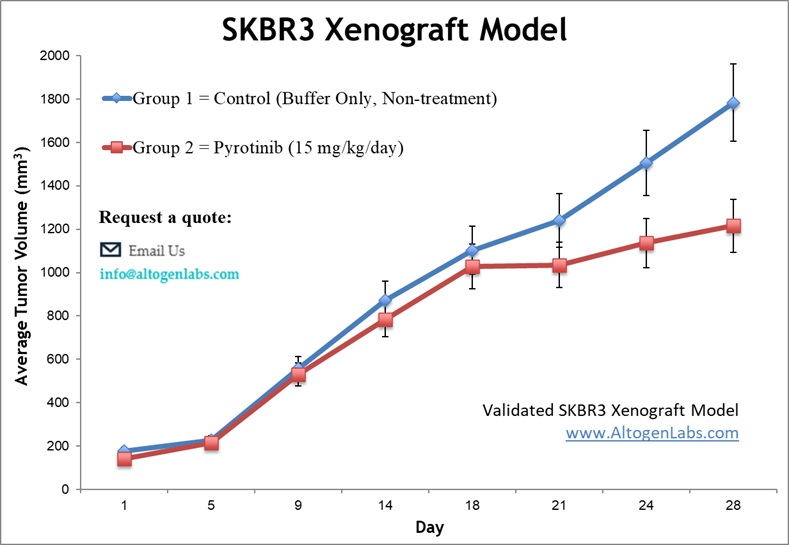
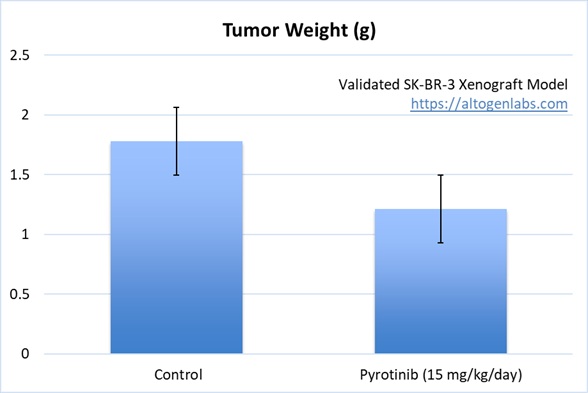
SK-BR-3 breast cancer cells are cultured under sterile conditions and assessed for viability (typically >95%) using assays such as trypan blue exclusion or flow cytometry. The cells are then subcutaneously injected into the flanks of 10-12-week-old athymic BALB/c or NOD/SCID mice, with each injection consisting of 1 x 10^6 SK-BR-3 cells suspended in a 1:1 mixture with Matrigel to facilitate tumor establishment. Tumor growth is monitored using digital calipers, and the health of the mice is tracked by regular body weight measurements. Upon reaching tumor sizes of approximately 75-150 mm³, the animals are randomly assigned to treatment groups, and tumor volume measurements are taken daily to evaluate the therapeutic response. At the conclusion of the study, necropsy is performed, and tumors are excised, weighed, and preserved for further analysis.
Structural Variations and Oncogene Fusions in the SK-BR-3 Cell Line
The SK-BR-3 cell line, a widely used model for HER2-positive breast cancer, exhibits extensive oncogene amplifications and complex genomic rearrangements. One of its most prominent oncogenes, ERBB2 (HER2), is highly amplified, with approximately 33 copies per cell, contributing to its aggressive phenotype. This amplification is driven by complex nested duplications, translocations, and an inverted duplication, suggesting a punctuated evolution rather than a gradual accumulation of mutations. Additionally, MYC and EGFR are also amplified, further promoting oncogenic signaling pathways. Long-read sequencing has revealed novel gene fusions, including those involving ERBB2, which may impact cancer progression and therapeutic resistance. Structural variations, such as inversions, translocations, and large insertions, are abundant and contribute to the instability of the SK-BR-3 genome.
Altogen Labs offers a robust platform for breast cancer research through its specialized SK-BR-3 xenograft models, designed to study tumor progression, therapeutic efficacy, and the underlying biological mechanisms of HER2-positive breast cancer. These models enable researchers to investigate tumor growth dynamics, treatment responses, and resistance mechanisms using quantitative methods such as Tumor Growth Delay (TGD) and Tumor Growth Inhibition (TGI) assessments. Researchers can assess the efficacy of novel therapies with flexible dosing strategies, including intravenous, oral gavage, or subcutaneous injections, tailored to specific treatment regimens. Advanced analytical techniques, including immunohistochemistry, gene expression profiling, and fluorescence-based imaging for in vivo tumor monitoring, provide detailed insights into tumor morphology, molecular pathways, and therapeutic responses. This comprehensive approach allows for the development and testing of targeted therapies for HER2-positive breast cancer and contributes to a deeper understanding of treatment mechanisms and drug resistance.
Evaluating HER2 Therapies Using the Subcutaneous SK-BR-3 Xenograft Model
The subcutaneous SK-BR-3 model is a well-established preclinical system used to study HER2-positive breast cancer. In this model, SK-BR-3 cells, derived from a human metastatic breast cancer, are implanted under the skin of immunocompromised nude mice, typically athymic BALB/c or NOD/SCID mice. This model is particularly useful for evaluating tumor growth, metastasis, and therapeutic responses to HER2-targeted treatments such as trastuzumab (Herceptin) and novel drug candidates. Tumor progression is monitored by regular measurements with digital calipers, providing valuable data on tumor volume and response to therapy. The subcutaneous SK-BR-3 model is also ideal for studying mechanisms of resistance and evaluating the efficacy of combination therapies. Additionally, molecular analysis of tumor tissue via immunohistochemistry or gene expression profiling allows for in-depth exploration of tumor biology and treatment effects. This model is typically used in preclinical testing for both conventional and experimental treatments in a controlled in vivo environment, mimicking clinical scenarios for HER2-positive breast cancer.
Orthotopic SK-BR-3 Model for Studying HER2-Positive Breast Cancer
The orthotopic SK-BR-3 model is a powerful preclinical tool used to study HER2-positive breast cancer in a more physiologically relevant context. In this model, SK-BR-3 cells, derived from a human breast carcinoma, are implanted into the mammary fat pad of immunocompromised mice, such as athymic BALB/c or NOD/SCID strains. This approach more closely mimics the natural tumor microenvironment and allows researchers to assess tumor growth, local invasion, and metastasis. The model is frequently employed to evaluate the efficacy of HER2-targeted therapies like trastuzumab (Herceptin) and newer drug candidates in a setting that reflects clinical tumor behavior. Tumor size and progression are monitored regularly, and detailed analysis can be performed through imaging techniques and histopathological examination. The orthotopic SK-BR-3 model also serves as an effective system for studying the molecular mechanisms underlying tumor metastasis, chemoresistance, and immune evasion.
Potential of Tolfenamic Acid as a Therapy for HER2-Positive Breast Cancer
A study conducted by Liu X, et al., published by Molecular Cancer Therapeutics journal demonstrated that tolfenamic acid (TA), a nonsteroidal anti-inflammatory drug (NSAID), effectively inhibits the growth of SK-BR-3 and BT474 breast cancer cells by downregulating erbB2 (HER2) expression. Unlike its effects in pancreatic cancer, TA does not target Sp transcription factors but instead reduces erbB2 mRNA stability and promoter activity, leading to decreased erbB2 protein levels. This downregulation inhibits downstream kinase signaling, including MAPK and Akt phosphorylation, and leads to increased p27 and decreased cyclin D1, ultimately suppressing cell proliferation. In xenograft models, TA treatment significantly reduces tumor growth and erbB2 expression, suggesting its potential as a novel, low-toxicity therapy for HER2-overexpressing breast cancers. TA’s ability to modulate key oncogenic pathways suggests its viability as a therapeutic option for HER2-positive breast cancer patients, especially those resistant to existing treatments like trastuzumab.
Estrogen-Induced SIRT1 Activation Enhances SK-BR-3 Tumor Growth
The role of SIRT1, a histone deacetylase, in SK-BR-3 breast cancer cells is explored, particularly its regulation through GPER (G-protein-coupled estrogen receptor) signaling. Unlike estrogen receptor-positive breast cancers, SK-BR-3 cells rely on GPER-mediated pathways, where estradiol (E2) and G-1 (a GPER agonist) upregulate SIRT1 via the EGFR/ERK/c-fos/AP-1 transduction pathway. This activation promotes cancer cell survival, proliferation, and resistance to DNA damage, preventing apoptosis triggered by etoposide treatment. Moreover, SIRT1 inhibition via Sirtinol or GPER silencing blocks these oncogenic effects, highlighting their interplay in tumor progression. In xenograft models, ligand-activated GPER enhances tumor growth through SIRT1-mediated mechanisms. These findings underscore SIRT1 as a key player in ER-negative breast cancer progression, suggesting that targeting GPER-SIRT1 signaling could be a promising therapeutic strategy.
Advancing Cancer Drug Testing with Patient-Derived Tumor Organoids
Organoids are three-dimensional cultures derived from patient tumor samples that maintain key features of the original tumor, such as genetic and phenotypic diversity. In contrast to traditional 2D cell cultures, organoids preserve complex tissue architecture and can be efficiently expanded from primary patient material, making them invaluable for personalized cancer research and drug testing. Unlike xenograft and allograft models, which involve implanting tumors into animals and enable tumor-stroma and immune interactions, organoids provide a faster, more scalable platform for assessing therapeutic responses in vitro. They offer the unique advantage of mimicking the native tissue microenvironment while being more efficient and reproducible than animal models. Recent advancements in organoid technology have facilitated the development of patient-derived tumor organoid (PDTO) biobanks, which act as living resources for investigating cancer progression, drug resistance, and personalized medicine strategies. These models are especially effective in high-throughput drug screening, allowing researchers to identify potential treatments tailored to individual tumor profiles. The use of organoids also enables the exploration of novel drug combinations and precision therapies with a greater understanding of tumor heterogeneity. Furthermore, organoids are ideal for studying mechanisms of drug resistance, offering insights into why certain treatments may fail in specific patient populations. By incorporating patient-specific variations, organoid models provide a more accurate representation of clinical outcomes, advancing cancer research and treatment development.
