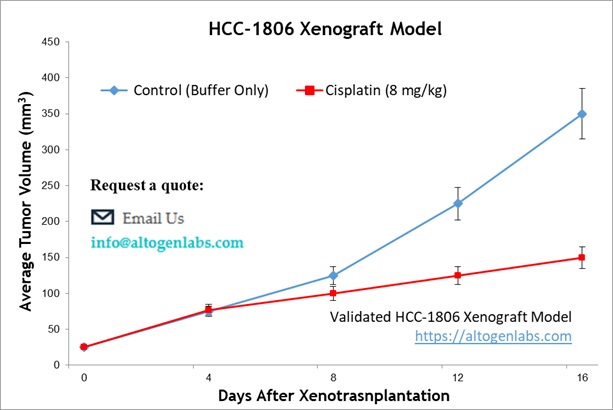
HCC-1806 Breast Cancer Metastatic Xenograft Model: Download ![]()
The ACS estimates for 2019 US breast cancer cases are: Approximately 268,600 new invasive cancer cases, 62,930 cases of breast carcinoma in situ (CIS, non-invasive early stage) and 41,760 deaths attributed to breast cancer. Recent years have shown a slight increase in breast cancer rates (+0.4%/year). The HCC 1806 cell line was derived from the stage II (no lymph node metastasis) primary cancer of a 50yo Black female diagnosed with squamous cell breast cancer. These cells are acantholytic, meaning that they do not have intercellular connections such as desmosomes. Squamous cell breast carcinoma is very rare, about 0.1% of all breast cancers. The HCC 1806 model has been used in many breast cancer research studies. Martin et al. published a 2017 Breast Cancer Research article using HCC 1806 xenografts to investigate the combination therapy of gefitinib, an EGFR inhibitor, with Fingolimod, also known as FTY720, the sphingosine kinase (SphK) inhibitor. Results demonstrated a loss of T-cell immunoreactivity, increased apoptosis, and suppression of EGFR phosphorylation and Ki67. Data also suggested that efficacy in targeting IGFBP-3-dependent pathways in basal-like breast cancer can be predicted with IGFBP-3 and CD44 biomarkers. In 2018, Liu et al. published a Scientific Reports study using HCC1806 and other cell lines to evaluate mithramycin A, an Sp1 inhibitor, in triple negative breast cancer. Data showed treatment caused apoptosis and proliferation inhibition with downregulated KLF5. This supports the use of MIT in TNBC by inhibiting the Sp1/KLF5 axis. Lastly, a 2012 Neoplasia study (Volk-Draper et al.) used HCC1806 cells to identify basal TNBC markers and establish a subline, HCC1806-RR, that expressed RFP and Renilla luciferase. Results demonstrated sensitivity to paclitaxel (accompanied by pro-survival VEGF-A loop), bevacizumab, and taxane therapy. Data also suggested that the established subline HCC1806-RR was suitable for quantifying metastasis due to B-TNBC markers. The HCC 1806 cell line is used to create the CDX (Cell Line Derived Xenograft) HCC 1806 xenograft mouse model. The HCC 1806 xenograft model has been used to investigate triple negative breast cancer characteristics and treatments.
HCC-1806 Cell Line
The HCC-1806 cell line is an epithelial cell line derived from the mammary gland of a 60-year-old Black female patient diagnosed with acantholytic squamous cell carcinoma (ASCC). This tumor was classified as TNM Stage IIB, grade 2. The cell line was initiated in 1995 and took approximately 10 months to fully establish in culture. HCC-1806 cells are often utilized in research focused on understanding breast cancer biology, particularly the molecular mechanisms underlying ASCC. The cell line provides a valuable insight for investigating therapeutic strategies for squamous breast cancer, a rare and aggressive form of the disease. Researchers commonly use HCC-1806 cells in xenograft studies to explore drug responses and to model tumor progression. Its unique characteristics, including high expression of epithelial markers and a distinct histological profile, make it a useful model for studying the heterogeneity of breast cancer subtypes. Additionally, HCC-1806 cells are known for their aggressive growth pattern, which makes them a useful model for studying tumor invasiveness and metastasis. Their utility in preclinical drug testing and their relevance to the study of rare breast cancer subtypes continue to make HCC-1806 an important resource in cancer research.
Altogen Labs Validated HCC-1806 Xenograft Model
Following expansion under aseptic conditions, HCC-1806 cells are harvested and prepared for injection while maintaining a minimum of 98% cell viability, verified by trypan blue exclusion assay. The cell suspension is adjusted to an appropriate density, ensuring that each immunocompromised mouse (e.g., athymic BALB/c or NOD/SCID, 10-12 weeks old) receives a single subcutaneous injection of 1 × 10⁶ HCC-1806 cells suspended in 100 µL of a Matrigel-cell mixture. Tumor growth is monitored using digital calipers three times weekly until tumors reach an average size of 75-125 mm³, at which point treatment administration begins. Animals are randomized into treatment and control cohorts, and test compounds are administered according to a pre-established dosing schedule. Body weights are recorded three times weekly, while tumor volumes are measured daily. The study endpoint is reached when tumors reach 2,000 mm³ or the predetermined size limit as per IACUC-approved protocols. At termination, animals are humanely euthanized, and tumors are excised, weighed, and documented using digital imaging. Collected tissues undergo downstream processing, including snap freezing in liquid nitrogen, stabilization in RNA-Later, or fixation for histological analysis.
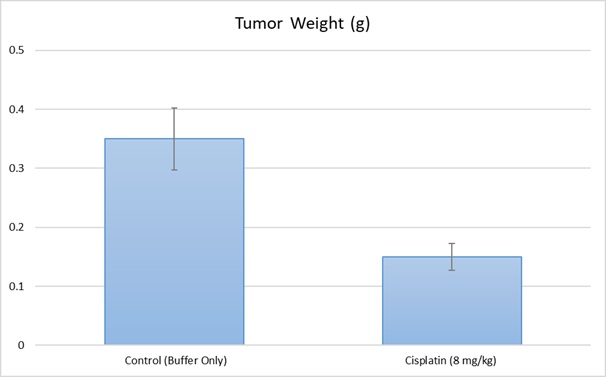
Xenograft models, including cell line-derived xenografts (CDXs) and patient-derived xenografts (PDXs), are critical for assessing the efficacy of novel cancer therapies. The HCC-1806 xenograft model specifically aids in the study of triple-negative breast cancer (TNBC), an aggressive subtype lacking targeted therapies. CDX models provide a highly reproducible system for tumor growth kinetics and drug response evaluations, whereas PDX models preserve the heterogeneity of patient tumors. At Altogen Labs, xenograft studies are conducted under GLP-compliant conditions following IACUC regulations. Mice undergo acclimation and are carefully monitored for tumor progression and clinical signs. We provide comprehensive experimental services, including histopathological evaluation, gene expression analysis (RNA/protein isolation), and customized dosing regimens. Our facilities are also equipped to support specialized diets and water systems for inducible gene expression research, ensuring precise and reliable preclinical data for oncology drug development.
Mitochondrial Dysfunction and ROS Generation in GA-Induced HCC-1806 Cell Apoptosis
HCC-1806 is a basal-like triple-negative breast cancer (TNBC) cell line used to investigate the therapeutic effects of gallic acid (GA) on TNBC progression. Research has shown that GA treatment significantly inhibited HCC-1806 cell proliferation and induced apoptosis by modulating key signaling pathways. Specifically, GA suppressed the PI3K/AKT/EGFR pathway, which is known to promote tumor cell survival and resistance to apoptosis. Concurrently, it activated the MAPK signaling cascade, including JNK and p38 pathways, leading to increased pro-apoptotic signaling. GA-induced apoptosis was associated with mitochondrial dysfunction, as evidenced by mitochondrial membrane potential depolarization and increased reactive oxygen species (ROS) generation. Molecular analyses demonstrated that GA upregulated Bax, cleaved caspase-3, and p53 while downregulating anti-apoptotic proteins such as Bcl-2 and phosphorylated EGFR. Additionally, computational molecular docking confirmed GA’s strong binding affinity for PI3K, AKT, and EGFR, further supporting its role in inhibiting these oncogenic drivers. These findings highlight the potential of GA as a promising natural compound for TNBC treatment, particularly in patients with elevated PI3K/AKT/EGFR signaling activity.
Nitroxidative Stress and Mitochondrial Dysfunction in HCC-1806 Cold Plasma Therapy
Research has used the HCC-1806 cell line used to evaluate the therapeutic potential of cold atmospheric plasma (CAP) in breast cancer treatment and found that CAP exposure significantly reduced HCC-1806 cell viability by inducing apoptosis and necrosis, with mitochondrial membrane potential loss being a key early event in cell death. The treatment also led to an increased BAX/BCL2 ratio and decreased procaspase-3 expression, confirming apoptotic pathway activation. Additionally, HCC-1806 cells exhibited unique nitroxidative stress responses, including elevated intracellular nitric oxide (NO) levels and reduced superoxide concentrations, suggesting an intrinsic ability to metabolize plasma-derived reactive nitrogen species. Inhibition of cytochrome c oxidase further enhanced CAP-induced cytotoxicity, indicating that oxidative stress modulation could potentiate plasma therapy. CAP also induced cell cycle arrest in the G2/M phase, impairing HCC-1806 proliferation and long-term survival. These findings highlight CAP’s potential as an innovative non-invasive therapeutic strategy for TNBC, particularly in tumors resistant to conventional therapies.
HCC-1806 Subcutaneous Xenografts
The subcutaneous HCC-1806 xenograft model is a well-established preclinical system for studying triple-negative breast cancer (TNBC). In this model, HCC-1806 cells are injected subcutaneously into immunocompromised mice, typically in the right flank, allowing for efficient tumor establishment and monitoring. Tumor growth is assessed using digital calipers, providing a reproducible and measurable system for evaluating therapeutic responses. This model is widely used to test novel anti-cancer compounds, including small molecules, biologics, and combination therapies. The subcutaneous approach offers a controlled tumor microenvironment, making it ideal for pharmacokinetic and pharmacodynamic studies. While it does not fully replicate the tumor’s natural site, it remains essential for drug efficacy screening. Study endpoints are determined based on tumor volume limits, with tissue collection enabling histological, molecular, and genomic analyses. The HCC-1806 subcutaneous model provides a reliable platform for investigating tumor biology and treatment responses in TNBC research.
Modeling Triple-Negative Breast Cancer Metastasis with HCC-1806
The metastatic HCC-1806 model is a valuable preclinical system for studying the dissemination and progression of triple-negative breast cancer (TNBC). This model is typically established through orthotopic implantation of HCC-1806 cells into the mammary fat pad of immunocompromised mice, allowing for spontaneous metastasis to distant organs such as the lungs, liver, and lymph nodes. It provides a clinically relevant platform for investigating metastatic mechanisms, tumor microenvironment interactions, and potential therapeutic interventions targeting metastatic TNBC. Researchers utilize this model to study gene expression changes associated with metastasis, evaluate novel anti-metastatic agents, and assess drug resistance in disseminated tumors. Imaging techniques such as bioluminescence or fluorescence tracking can be employed to monitor metastatic progression in vivo. The metastatic HCC-1806 model enables detailed histological and molecular analyses of metastatic lesions, facilitating the identification of new biomarkers and therapeutic targets. By mimicking key aspects of human TNBC metastasis, this model plays a critical role in advancing treatment strategies for aggressive and treatment-resistant breast cancer subtypes.
Oncogenic Drivers of HCC-1806: EMT, ECM Degradation, and Epigenetic Dysregulation
HCC-1806 is a basal-like triple-negative breast cancer (TNBC) cell line characterized by aggressive tumorigenic properties and genomic instability. A key oncogenic feature of HCC-1806 is the dysregulation of epithelial-to-mesenchymal transition (EMT) pathways, which enhance its invasive potential. This cell line exhibits high matrix metalloproteinase (MMP) activity, particularly MMP-2 and MMP-9, which degrade the extracellular matrix (ECM) and facilitate metastasis. Additionally, HCC-1806 harbors mutations in TP53, a tumor suppressor gene frequently altered in TNBC, leading to defects in apoptosis and genomic maintenance. Epigenetic alterations, including EZH2 overexpression, contribute to transcriptional silencing of key tumor suppressor genes, further driving malignancy. The PI3K/AKT and JAK/STAT signaling pathways are hyperactivated in HCC-1806, supporting its survival, proliferation, and resistance to therapy. Recent studies highlight the role of long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) in modulating HCC-1806 oncogenesis, with miR-200 family members being notably downregulated, promoting EMT and stemness. Understanding these oncogenic drivers provides insights into potential therapeutic targets for basal-like TNBC.
Basic Study Design
- HCC-1806 cells are maintained in exponential growth phase under aseptic conditions
- Cells are trypsinized and cell count viability is determined using a trypan blue exclusion assay (98% of cell viability is required).
- Each mouse is singly subcutaneously injected into the right flank with 1-2 x 106 cells in 100 µL of a Matrigel w/ HCC-1806 cell suspension
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 75-125 mm3 as measured via digital calipers
- Animals are randomized into treatment groups. Administration of test compound is performed according to the pre-established treatment schedule
- Mice weights are measured and recorded 2-3 times weekly
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
