Validated CAL51 Xenograft Tumor Model: Download ![]()
Breast cancer, the most common malignancy in women, presents with symptoms such as lumps, skin dimpling, and nipple changes, often influenced by genetic mutations like BRCA1 and BRCA2. Diagnosis relies on biopsy and classification based on histopathology, receptor status (ER, PR, HER2), and tumor staging, which guide treatment decisions. Targeted therapies like tamoxifen and trastuzumab are tailored to receptor profiles, while treatment strategies include chemotherapy, radiation, and hormone therapy. Human xenograft models, including breast cancer-derived models, are essential for studying tumor behavior and evaluating drug efficacy. These models are selected based on tumor morphology, genetic profile, and disease stage to closely replicate human breast cancer. Altogen Labs offers diverse breast cancer xenograft models to support preclinical research and therapeutic development.
CAL51 Cell Line
The CAL51 cell line is a human breast cancer cell line derived from a malignant pleural effusion in a woman diagnosed with metastatic breast cancer. This cell line is classified as triple-negative, meaning it lacks the expression of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2), making it an important model for studying aggressive and difficult-to-treat breast cancers. CAL51 cells are highly invasive, exhibiting strong migratory and metastatic properties, which makes them useful for research into cancer metastasis and progression. They are also known to be resistant to certain conventional chemotherapy agents, which further highlights their relevance in studying therapeutic resistance. CAL51 cells maintain several characteristics of the basal-like subtype of breast cancer, making them a representative model for investigating this subtype, which is typically associated with poor prognosis. These cells have been utilized in a wide variety of studies, including drug screening, molecular signaling pathways, and the development of targeted therapies. Due to the aggressive nature and molecular profile, CAL51 cells are valuable in investigating potential therapeutic strategies for triple-negative breast cancer, a subtype with few effective treatment options.
Get Instant Quote for
CAL51 Xenograft Model
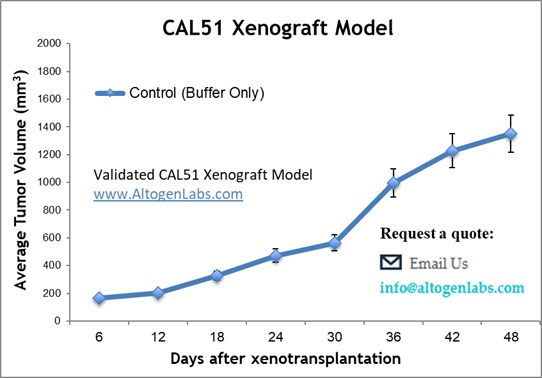
Altogen Labs Validated CAL51 Xenograft Model
At Altogen Labs, CAL51 cells are maintained in the exponential growth phase under aseptic conditions. The cells are trypsinized, and viability is assessed using a trypan blue exclusion assay, ensuring a cell viability rate of at least 98-99%. The CAL51 cell suspension is then adjusted to the appropriate density for injection. Each mouse receives a single subcutaneous injection of 1 x 10⁶ cells in 100 µL of the cell suspension, administered into the right flank. The injection sites are palpated up to three times per week until tumors reach an average size of 50-150 mm³, as measured using digital calipers. Once tumors are established, animals are randomized into treatment groups, and the test compounds are administered according to a pre-established treatment schedule. Mouse body weights are recorded three times weekly, while tumor measurements are taken daily. The study concludes when tumors reach 2,000 mm³ or the predetermined size limit as specified in the approved IACUC protocol. At the end of the study, a final necropsy is performed, and tissues are collected for downstream analysis.
Targeting Aurora Kinase A in CAL51
CAL-51, a triple-negative breast cancer (TNBC) cell line, was investigated in a study by Tentler JJ, et al., published by Molecular Cancer Therapeutics journal, to understand its response to Aurora kinase A (AurA) inhibition via alisertib. This kinase plays a crucial role in mitosis, and its overexpression is linked to TNBC progression. The study demonstrated that alisertib effectively induced apoptosis in CAL51, but its efficacy was dependent on the presence of functional p53 and p73. Knockdown of these tumor suppressors in CAL51 shifted the cellular response from apoptosis to senescence, suggesting an alternative resistance mechanism. Furthermore, xenograft models derived from CAL51 confirmed that long-term exposure to AurA inhibitors led to the emergence of resistant tumors exhibiting senescence. These findings emphasize the critical role of p53 family members in dictating therapeutic outcomes in TNBC and suggest that p53 status could serve as a biomarker for response to AurA inhibitors. The study highlights the need for combination therapies to prevent resistance by targeting both apoptosis and senescence pathways in aggressive TNBC models like CAL51.
Subcutaneous CAL51 Lung Cancer Xenograft Model
In the subcutaneous CAL51 model, CAL51 cells are subcutaneously injected under the skin of immunocompromised mice, where they grow into solid tumors. This model allows for easy monitoring of tumor growth through external measurements, making it ideal for evaluating the effects of therapeutic interventions on tumor size and progression. The subcutaneous CAL51 xenograft model mimics certain characteristics of human breast cancer, including its rapid growth and resistance to conventional therapies, which makes it particularly useful for testing new treatments and understanding resistance mechanisms. Researchers have used this model to assess the efficacy of chemotherapies, targeted therapies, and immunotherapies. Due to its ability to simulate human tumor biology, the model is also employed in the investigation of cancer cell proliferation, apoptosis, and molecular signaling pathways. Often times, the subcutaneous model is used to explore the potential of combination therapies and the effects of the tumor microenvironment on cancer progression. The subcutaneous CAL51 model is considered a reliable and reproducible system for preclinical drug development, particularly for testing strategies targeting triple-negative breast cancer.
Exploring Tumor Growth in the CAL51 Orthotopic Mouse Model
In the CAL51 orthotopic model, CAL51 cells are injected directly into the mammary fat pad of immunocompromised mice, closely mimicking the tumor’s natural microenvironment and growth pattern as it would occur in human breast tissue. This orthotopic setup allows for the observation of tumor growth, invasion, and metastatic spread in a manner that more accurately reflects the clinical presentation of triple-negative breast cancer (TNBC). The CAL51 orthotopic model has been used extensively to test novel therapeutic approaches, including targeted therapies and immunotherapies, due to its ability to replicate the heterogeneity and resistance commonly seen in human TNBC. Studies utilizing this model have highlighted the potential of combining EGFR-targeted treatments with inhibitors of cell cycle regulators, such as CDK2, as a way to enhance therapeutic efficacy. Furthermore, the model is valuable for investigating tumor progression, as it enables researchers to study both primary tumor growth and metastatic dissemination to distant organs. Due to its aggressive nature, the CAL51 orthotopic model is also instrumental in exploring tumor-stroma interactions and the role of the tumor microenvironment in influencing therapy response.
The Role of Subtle Genetic Changes in the Aggressive Tumorigenesis of CAL51
CAL51 is a unique triple-negative breast cancer (TNBC) cell line distinguished by its normal diploid karyotype, an uncommon feature among breast cancer models. Unlike most TNBC cell lines, which typically exhibit extensive chromosomal aberrations, CAL51 maintains a stable karyotype with 46 chromosomes and no detectable structural abnormalities, even under high-resolution banding. Cytogenetic analyses confirmed the absence of recurrent chromosomal alterations, suggesting that the oncogenic transformation of CAL51 occurred without major karyotypic disruptions. However, a subset of metaphases displayed non-clonal anomalies, such as sporadic chromosome gains or losses, indicating a degree of chromosomal instability. Interestingly, despite its normal karyotype, CAL51 retains robust tumorigenic potential, forming aggressive xenografts in nude mice. The stability of its chromosomal composition makes CAL51 a valuable model for studying tumorigenesis mechanisms that do not rely on gross chromosomal alterations, potentially highlighting the role of epigenetic or subtle genetic changes in driving malignancy.
Overcoming Treatment Resistance in CAL51 with EGFR-Targeted Antibody-Drug Conjugates
CAL51 is a highly aggressive triple-negative breast cancer (TNBC) cell line characterized by its oncogenic properties, including rapid proliferation, genomic instability, and resistance to standard therapies. It exhibits overexpression of EGFR, a key driver of tumor growth and survival, alongside dysregulation of the cell cycle, particularly through the CDK2/cyclin E axis. The dysregulation of these pathways contributes to treatment resistance in TNBC, emphasizing the need for targeted approaches. Researchers explored a novel antibody-drug conjugate (ADC) strategy using cetuximab, an anti-EGFR antibody, conjugated with a CDK inhibitor to selectively deliver cytotoxic agents to EGFR-expressing cells. The ADC efficiently internalized into tumor cells, inhibited cell cycle progression, and exhibited potent tumor-restricting effects in vitro and in vivo, including against chemotherapy-resistant TNBC models. Additionally, the ADC demonstrated bystander killing effects, eradicating EGFR-low neighboring cells, which may help overcome tumor heterogeneity. These findings suggest that targeting EGFR alongside dysregulated cell cycle pathways could provide a promising therapeutic strategy for aggressive and treatment-refractory TNBCs like CAL51.
Altogen Labs, based in Austin, Texas, is a leading contract research organization (CRO) specializing in xenograft studies for oncology research, including the use of CAL51 cells. With extensive experience in human tumor xenograft models, the company supports both preclinical and clinical research aimed at advancing cancer therapies. The services include tailored study design, selection of appropriate cancer models or cell lines, optimization of host animal selection, and both subcutaneous and orthotopic xenografting techniques. Altogen Labs also provides daily monitoring of experimental subjects and post-study analysis, including serum collection and histological examination. The CAL51 xenograft models are particularly valuable for studying the aggressive nature of triple-negative breast cancer (TNBC), including tumor growth, metastasis, and drug resistance. These efficacy studies xenograft models allow for the evaluation of experimental treatments and the study of the mechanisms underlying TNBC’s resistance to conventional therapies. Altogen Labs offers the specialized expertise to support research into novel treatment strategies for aggressive breast cancer subtypes like CAL51 using in vivo xenograft models.
