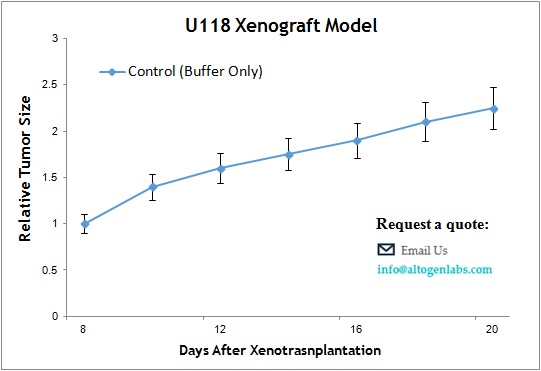
U118 Brain Cancer Xenograft Model
Glioblastoma, or glioblastoma multiforme (GBM), is an aggressive brain cancer with symptoms including nausea, personality changes, headaches, or stroke-like symptoms. Causes of glioblastoma remain unclear, although rare genetic disorders can be risk factors. Usual onset is above 64 years of age. Temozolomide and steroids are common drug treatments; surgery and radiation are also used. The U118 cell line was derived from a 50 y.o. Caucasian male diagnosed with astrocytoma. The U118 model has been used in many glioblastoma research studies. A 2008 Journal of Translational Medicine article (Xie et al.) used various glioblastoma cell lines, including U118, in order to generate invasive orthotopic xenografts optimized for real-time ultrasounds of tumors so that anti-neoplastic drugs such as 17AAG may be evaluated. The group used experimental lung metastasis (ELM) assays in select for the most metastatic/aggressive cells for the use in xenografts; resulting orthotopic cells had vascular hyperplasia, intracranial dissemination and areas of central necrosis.
A 2004 Oncogene article by Zhang et al. used the U118 model in their study of the hepatocyte growth factor (HGF)/scatter factor (SF) interaction with Met in xenografts. Typical xenografts do not replicate HGF/SF-mediated stimulation of the paracrine system, and the results suggested that ectopically expressing human HGF/SF can enhance subcutaneous xenograft growth. The U118 cell line is used to create the CDX (Cell Line Derived Xenograft) U118 xenograft mouse model. The U118 xenograft model has been used to investigate glioblastoma characteristics and therapies.
U118 xenograft models can be used to study the biology of glioblastoma, including the mechanisms of tumor growth and metastasis, as well as the tumor’s response to various treatments such as chemotherapy, radiation therapy, and immunotherapy. These models can also be used to evaluate the efficacy and safety of new brain cancer therapies before testing them in human clinical trials.
U118 Brain Cancer Xenograft Model: Download ![]()
U118 Cell Line
The U118 glioblastoma cell line, derived from human astrocytoma, is widely used as a preclinical model to investigate the molecular biology and therapeutic responses of glioblastoma multiforme (GBM). It is defined by the loss of PTEN expression, a mutant p53 genotype, and constitutive activation of the PI3K/AKT signaling pathway, all of which are features commonly associated with GBM progression and resistance to therapy. U118 cells display a mesenchymal phenotype and express markers such as CD44 and YKL-40, which are linked to increased invasiveness and poor clinical outcomes. Although the cell line shows responsiveness to some chemotherapeutic agents, it exhibits relative resistance to temozolomide (TMZ), reflecting the treatment challenges faced in clinical settings. While U118 is frequently employed in monotherapy screening and standard in vitro assays, it remains underutilized in studies exploring combination therapies or epigenetic modulation. Moreover, its application in orthotopic xenograft modeling and investigations of tumor microenvironment interactions is limited. These gaps underscore the need for further research to clarify the value of U118 in modeling drug resistance and testing novel therapeutic approaches.
Modeling Mesenchymal GBM Using U118 Orthotopic Transplants
Orthotopic xenograft models provide a clinically relevant platform for studying glioblastoma multiforme (GBM) by replicating the native brain microenvironment, including tumor-stroma interactions, vascular architecture, and therapeutic barriers such as the blood-brain barrier. Although U118 glioblastoma cells have traditionally been used in subcutaneous models, they have also been successfully adapted for orthotopic transplantation via stereotactic injection into the brains of immunodeficient mice. U118 cells, which harbor PTEN loss, p53 mutation, and PI3K/AKT pathway activation, represent the mesenchymal subtype of GBM and are suitable for modeling resistant tumor phenotypes. Recent studies utilizing luciferase-tagged U118 cells (U118-Luc) have enabled real-time, noninvasive tracking of intracranial tumor growth and demonstrated the effectiveness of experimental therapeutics, including targeted fusion proteins and radiosensitizers. These findings reinforce the value of U118 orthotopic xenografts in evaluating drug efficacy within a more representative physiological context and support their broader use in translational GBM research.
Evaluating Therapeutic Response in U118 Glioblastoma Models
The U118 glioblastoma cell line offers a relevant model for investigating mesenchymal glioblastoma, especially in the context of gene-based therapies and radio-sensitization strategies. Characterized by PTEN deletion and p53 mutation, U118 cells exhibit aberrant activation of the PI3K/AKT pathway and are notably resistant to conventional treatments. When evaluated in vitro, U118 cells demonstrate sensitivity to certain targeted agents that inhibit pathways involved in proliferation and angiogenesis, such as MET and VEGFR2. Treatment results in reduced cell viability, induction of senescence, and suppression of key proliferative regulators including cyclin D1, CDK4, and phosphorylated Rb. Senescence is further supported by increased β-galactosidase activity and upregulation of p16. Notably, the combination of targeted therapy with ionizing radiation significantly enhances cell death, indicating a synergistic effect and potential for overcoming intrinsic radio-resistance.
Orthotopic xenograft models using luciferase-tagged U118 cells allow real-time monitoring of tumor progression within the brain and enable precise assessment of therapeutic impact. In vivo results show that effective gene delivery to intracranial tumors can reduce tumor proliferation, suppress angiogenesis, and increase apoptotic activity. These effects are corroborated by decreased expression of Ki67 and CD31, as well as increased TUNEL-positive cells within tumor sections. Despite the promising biological outcomes, limitations such as small sample sizes and the exclusive use of a single cell line underscore the need for broader validation across glioblastoma subtypes. Nonetheless, the U118 model remains instrumental in dissecting molecular mechanisms
U118 Glioblastoma Models Reveal Microenvironment-Driven Changes
A study by Creighton et al., published in Genome Biology journal, presents a comprehensive comparison of gene expression profiles between cultured U118 glioblastoma cells and their subcutaneous xenograft tumors in immunodeficient mice. Using Affymetrix microarrays, the researchers identified 368 genes with significant expression differences between in vitro and in vivo conditions (p < 0.01, fold change greater than 2). Among these, 112 genes were upregulated in xenografts and 256 in cultured cells. U118 xenografts showed increased expression of genes related to extracellular matrix remodeling, adhesion, cytokine activity, and angiogenesis, including COL1A1, COL1A2, COL5A2, PDGFRB, and ELN. In contrast, cultured U118 cells expressed higher levels of genes linked to cell cycle progression, DNA replication, and metabolic activity, reflecting a more proliferative state in standard culture environments. The study’s strength lies in its use of biological replicates, statistical rigor, and functional annotation to validate its findings. Principal component analysis confirmed that both in vitro and in vivo U118 profiles clustered with brain tumor datasets, supporting the model’s lineage fidelity. Interestingly, even when implanted in the same host environment, U118 and A549 cells exhibited distinct extracellular matrix expression patterns, suggesting cell-type-specific adaptations. Mouse RNA contamination was ruled out through control analyses, strengthening the conclusion that gene expression changes originated from human tumor cells. These findings emphasize the importance of xenograft models for capturing context-dependent gene regulation that is absent in vitro. The U118 xenograft model is particularly valuable for studying glioblastoma-associated processes such as extracellular matrix remodeling and angiogenesis.
U118 Resistance to TMZ Linked to Survival Pathway Activation
The U118 glioblastoma cell line provides a valuable model for studying resistance to temozolomide (TMZ), a standard chemotherapeutic agent used in glioblastoma treatment. Exposure of U118 cells to increasing concentrations of TMZ resulted in a clear reduction in proliferation, particularly at doses above 100 micromolar. Despite this growth inhibition, apoptosis levels remained low, and no significant cell cycle arrest in G1 or G2 phases was observed. Chromatin condensation and nuclear abnormalities confirmed limited apoptotic activity, while a marked increase in LC3 expression suggested that autophagy may serve as a compensatory survival mechanism. These results support the hypothesis that U118 cells evade TMZ-induced death by activating non-apoptotic pathways and maintaining survival signaling.
Further analysis revealed that U118 cells retained basal activation of both the PI3K/Akt and ERK1/2 MAPK pathways during TMZ treatment. Pharmacologic inhibition of these pathways using wortmannin, rapamycin, and U-0126, individually and in combination with TMZ, resulted in significant increases in apoptotic cell death. The most pronounced effect occurred when all three inhibitors were used in conjunction with TMZ, yielding a 71 percent apoptotic rate. This synergistic effect highlights the critical role of these signaling pathways in maintaining glioma cell survival. The methods employed, including flow cytometry, Western blotting, and proliferation assays, were robust and well controlled, although limited to in vitro conditions. These findings emphasize the need for integrated therapeutic approaches that target multiple survival mechanisms and warrant further investigation in orthotopic models to better replicate the brain tumor microenvironment.
U118 Brain Cancer Xenograft Model: Download ![]()
Basic Study Design
- U118 cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using a trypan blue exclusion assay (98% of cell viability is required). U118 cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 106 cells in 100 µL of a Matrigel- U118 cell suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 50-150 mm3 as measured via digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the established treatment schedule.
- Mice weights are measured and recorded 3 times weekly; tumors are measured and recorded daily.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNAlater, snap frozen in LN2 or prepared for histology.
