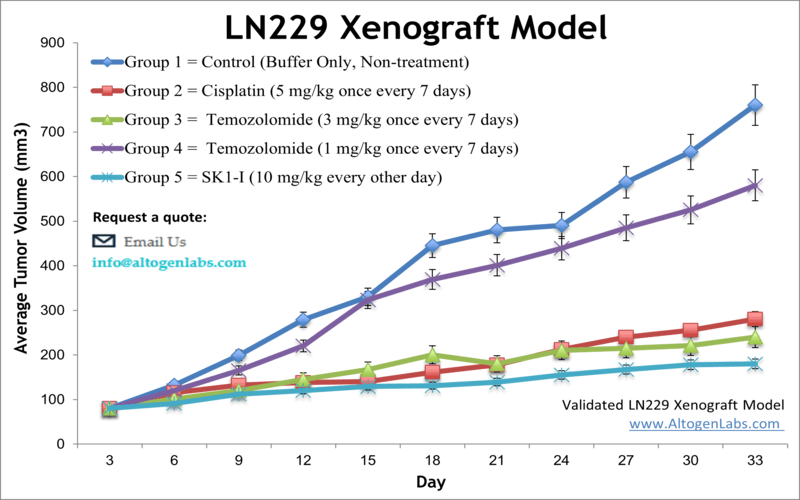
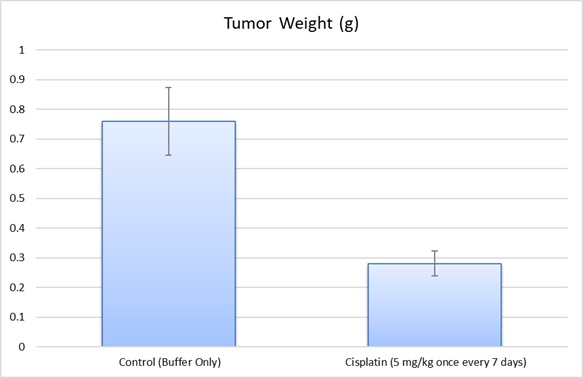
The LN-229 human brain glioblastoma cell line was first obtained in 1979 from a patient with right frontal parieto-occipital glioblastoma. The LN-229 cell line has a wild-type PTEN gene, mutated p53, and may potentially have homozygous deletions in the p16 as well as the p14ARF tumor suppressor genes. In a 1997 study, published in BBA Molecular Cell Research Journal, the LN-229 cell line was evaluated after the treatment with puromycin that killed LN-229 cells in a dose-dependent manner. In addition, stimulation of these cells with Fas ligand lead to apoptotic cell death within 16 hours. A 2011 Cancer Research study identified the LN-229 rodent xenograft model as a valid system to use the cryo-imaging technique for examining tumor progression and metastasis. This method differs from the traditional methods of serial sections and histological stains of brain tissue in that it uses computer algorithms to reconstruct 3-D images of this glioma model, essentially improving resolution and allowing the study of tumor cell invasion and dispersal. The LN-229 xenograft model has also been used in a 2013 study by Grommes et al. to identify the proliferator-activated receptor gamma (PRARγ) small molecule agonist pioglitazone as a potential therapeutic treatment for malignant gliomas. Pioglitazone was shown to cross the blood-brain barrier and exhibit antineoplastic effects in the LN-229 glioblastoma xenograft model. The 2012 study by Chen et al also used the LN-229 cell line; this group identified a brain specific micro-RNA, miR-524-5p, that acts as a tumor suppressor. They demonstrated the targeting of Jagged-1 and Hes-1, key components of stem cell maintenance and angiogenesis pathways, by miR-524-5p and subsequent suppression of cell proliferation and invasion upon the miRNA’s restoration. Altogen Labs provides xenotransplantation services for LN-229 glioblastoma cells to study tumor formation and progression. The LN-229 cell line is used to create the CDX (Cell Line Derived Xenograft) LN229 xenograft mouse model. Targeting S1P to induce apoptosis (e.g. SK1-I) and inhibit AKT signaling are some of the uses of the LN-229 xenograft model, including tumor growth suppression with chemotherapies (e.g. temozolomide or cisplatin).
LN229 Brain Orthotopic And Metastatic Xenograft Model: Download ![]()
Download Altogen Labs LN-229 Xenograft Model PowerPoint Presentation: ![]()
Get Instant Quote for
LN-229 Xenograft Model
Tumor Microenvironment in Glioblastoma Growth
Glioblastoma multiforme (GBM) is an aggressive brain cancer known for its invasive growth into surrounding brain tissue. The LN229 glioblastoma cell line is a valuable model for studying this invasive behavior, as it more closely mimics human GBM than other commonly used cell lines like U87. Unlike U87 cells, which form well-defined tumor margins, LN229 cells exhibit diffuse growth and migrate extensively along blood vessels, demonstrating their high invasive potential. This migration is influenced by interactions with the tumor microenvironment, particularly through the CXCR4-STAT3 signaling axis. LN229 cells respond strongly to CXCL12, a chemokine secreted by brain endothelial cells, which enhances their infiltration into brain tissue. Moreover, LN229 tumors recruit and polarize microglia and macrophages into an immunosuppressive M2-like state, supporting further tumor progression. These findings highlight LN229 as a crucial part for studying glioblastoma invasion and potential therapeutic targets that could disrupt tumor spread.
Investigating Glioblastoma Therapy with the Orthotopic LN229 Model
The orthotopic LN229 model is a widely used in vivo system for studying glioblastoma, where LN229 cells are implanted directly into the brain, mimicking the natural tumor environment. This model allows researchers to observe tumor growth, invasiveness, and interactions with the surrounding brain tissue, providing valuable insights into glioblastoma’s biology and pathology. By implanting the cells into the brain, the orthotopic model preserves the complex tumor microenvironment, including blood-brain barrier interactions and immune responses. The model is often utilized to evaluate the efficacy of novel therapeutic agents, such as targeted therapies and immunotherapies, in a setting that closely resembles clinical conditions. Additionally, the orthotopic LN229 model is employed to study tumor progression, including invasion into neighboring tissues and resistance to treatment, which is a key challenge in glioblastoma therapy. Researchers can also assess the effects of genetic modifications, such as overexpression or silencing of specific genes, to investigate the molecular drivers of glioblastoma progression. This model has proven to be a powerful tool for preclinical testing and the development of new therapeutic strategies.
Understanding Glioblastoma Metastasis with the LN229 Model
The metastatic LN229 model is an advanced preclinical tool used to study the spread of glioblastoma beyond its primary site. In this model, LN229 cells are typically injected into the bloodstream, often via the tail vein, to investigate the potential for metastatic dissemination to distant organs. Unlike primary brain tumors, metastatic models provide insights into how glioblastoma cells invade other tissues, including the lungs, liver, and bones, mimicking the complex biology of metastatic spread in human disease. Researchers utilize this model to understand the molecular mechanisms underlying glioblastoma metastasis, including the role of tumor microenvironment interactions and cell adhesion processes. The metastatic LN229 model is also instrumental in evaluating the effectiveness of novel therapies aimed at preventing or treating metastasis. By tracking tumor growth in distant organs, researchers can assess the therapeutic potential of drugs that target metastatic pathways. This model allows for a more comprehensive understanding of glioblastoma progression, providing a critical platform for evaluating combination therapies that target both primary and metastatic tumors.
Biguanides as Potential Therapeutic Agents for LN229 Glioma Cells
Glioblastoma is an aggressive brain cancer with poor treatment outcomes, making it essential to research and explore novel therapeutic approaches. A study by Wang Y, et al., published by OncoTargets and Therapy journal, investigated the effects of biguanides, specifically phenformin (Phen) and metformin (Met), on the LN229 glioma cell line. Both drugs significantly inhibited LN229 cell proliferation, induced cell cycle arrest, and promoted cell death through mitochondrial reactive oxygen species (ROS) imbalance. In vitro experiments demonstrated that Phen and Met reduced LN229 migration and colony formation, likely through changes in E-cadherin and vimentin expression. In vivo, treatment with Phen and Met effectively suppressed LN229 tumor growth and metastasis in a mouse xenograft model. The study also revealed that the antitumor effects of these drugs were ROS-dependent, as the ROS inhibitor NAC successfully rescued LN229 cells from death. Interestingly, while both drugs activated AMPK, an AMPK inhibitor did not reverse their cytotoxic effects, suggesting an AMPK-independent mechanism of action. These findings highlight Phen and Met as promising adjunct therapies for glioblastoma treatment, particularly for targeting highly invasive LN229 cells.
Targeting Acid Adaptation in LN229 Glioblastoma Cells
Glioblastoma is an aggressive brain cancer with complex cellular adaptations that support tumor survival and progression. The LN229 glioblastoma cell line has been instrumental in studying how extracellular acidity influences tumor behavior. Changes in proton concentrations in the tumor microenvironment affect the structure of surface lipids, such as cholesterol and GM3 glycosphingolipid, which in turn regulate cell survival, migration, and proliferation. At lower pH levels, LN229 cells undergo significant lipid remodeling, forming protective clusters that enhance resistance to harsh conditions. These adaptations promote oncogenic survival by preventing membrane degradation and supporting metabolic flexibility. Additionally, targeting key surface lipids in LN229 cells, such as GM3, with specific antibodies can mimic low pH conditions and induce differentiation or programmed cell death, providing a potential therapeutic strategy. The study of LN229 oncogenes in these acidic environments offers valuable insight into how glioblastoma cells evade treatment and continue to grow. Understanding these mechanisms could lead to new approaches that exploit tumor vulnerabilities and improve glioblastoma therapies.
Basic study design
- Exponentially growing cells are collected for inoculation with viability determined by trypan blue exclusion. A 99% minimum cell viability is required.
- Suspensions are adjusted so 100 µL of 50% Matrigel solution w/ LN229 cell suspension contains one million cells. Cells are inoculated s.c. into a hind leg per mouse. The mice are NOD.CB17-Prkdc or athymic BALB/C strain and 9-11 weeks old at the time of xenotransplantation.
- Calipers are utilized for tumor monitoring, with 150-200 mm3 size tumors needed to initiate the study.
- Tumor measurements and mouse weights are recorded until tumor size limits are reached.
- Necropsies are performed for tumor removal, weights are logged and digital images captured.
- Choices for tissue collection include: submersion in RNA-later, snap freeze, isolate nucleic acids or 10% NBF formalin storage to prepare for histological analysis.
Get Instant Quote for
LN-229 Xenograft Model
The LN229 is a cell line derived from a human glioblastoma, this cell line is commonly used in preclinical research to study the biology of glioblastoma and to test new treatments using LN-229 xenograft model. This is important because it can help to determine whether a treatment is safe and effective before it is tested in humans. A xenograft is a transplant of cells or tissue from one species to another (in medicine, it is often also used to refer to a tissue graft from a human to a non-human specie). Xenotransplantation is the process of transplanting tissue or organs from one species to another. Xenograft animal models are commonly used to test the effectiveness of drugs against specific types of cancer. All clinically approved anticancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the LN-229 xenograft model:
- LN-229 Tumor Growth Delay (TGD; latency)
- LN-229 Tumor Growth Inhibition (TGI)
- Flexible dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal; injections are performed using cutting-edge micro-injection techniques and pump-controlled IV injection)
- LN229 tumor immunohistochemistry (typically part of the preclinical Pharm/Tox studies)
- Alternative cell engraftment sites (orthotopic transplantation or tail vein injection and left ventricular injection for metastasis studies)
- Blood chemistry analysis (typically part of the preclinical Tox studies)
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology (typically part of the preclinical toxicology studies)
- Positive control group employing 20-50 mg/kg cisplatin administered daily for the study duration
Get Instant Quote for
LN-229 Xenograft Model
LN229 is a human glioblastoma cell line commonly used in brain cancer research. It was derived from a malignant glioblastoma tumor in a patient and is now widely used as a model system to study the biology of brain tumors and test potential cancer therapies. LN-229 cells have been extensively characterized and are known to harbor several genetic mutations, including mutations in the tumor suppressor gene p53 and the oncogene EGFR. These mutations are commonly found in glioblastoma tumors and are thought to contribute to tumor growth and resistance to therapy. LN229 cells are also notable for their ability to form tumors when injected into immunodeficient mice. This property makes them useful for studying the biology of glioblastoma tumors in vivo, and for testing potential cancer therapies in nonclinical models.
LN229 cells have been used in numerous xenograft studies to investigate the mechanisms of glioblastoma growth and metastasis, as well as to test the efficacy of various therapeutic strategies, including chemotherapy, radiation therapy, and immunotherapy. These studies have contributed to our understanding of the biology of glioblastoma and have helped to identify new targets for the development of more effective brain cancer treatments. Overall, LN229 is a valuable tool in brain cancer research, and its use has contributed to significant advances in our understanding of the disease and the development of new therapies.
LN229 Brain Orthotopic And Metastatic Xenograft Model: Download ![]()
