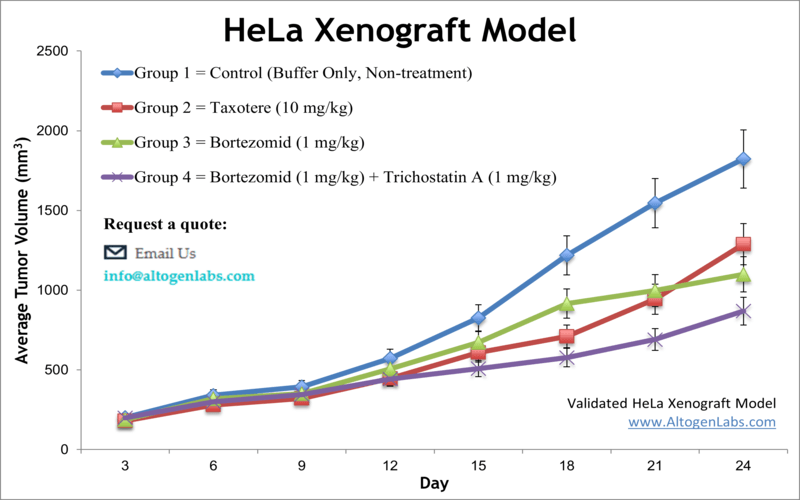
HeLa xenograft model
Cervical cancer is the second most common malignancy among females worldwide. The HeLa cell line was isolated from cervical cancer cells of a 31-year-old woman and is the first immortal human cell line that can survive indefinitely in vitro. HeLa is helpful for the prevention of aging and cell death because of active telomerase in cell divisions. Moreover, it was used to test the first polio vaccine. HeLa is essential for gene mapping as well as for AIDS and cancer research. A 2017 study by Liu et al. published in International Journal of Clinical and Experimental Medicine investigated the anticancer effect of Fluoxetine (FLX) alone or in combination with Cisplatin (DDP) in vitro on HeLa human cervical cancer cell and in vivo using the HeLa xenograft model. The article indicates that the combination of FLX and DDP significantly inhibits the proliferation of HeLa cells in a dose-dependent manner, inducing cell cycle arrest and apoptosis in vitro. Furthermore, the combination treatment suppressed the tumor growth in the HeLa xenograft mouse model which supports its potential as an effective therapeutic approach for cervical cancer patients. Another study by Liu et al. (2018, Food Science and Technology) used the HeLa xenograft model to study the anticancer effects and mechanism of curcumin (cur), a polyphenol found in turmeric roots. Results indicated that cur treatment led to inhibition of tumor growth and affected a multitude of protein expression levels including Bax, p53, p21, Bcl-2, HIF-1alpha, MIF and VEGF which are involved in cell cycle, apoptosis and angiogenesis, among other processes. This supports the use of cur as a potential chemotherapy agent for cervical cancer. Lastly, the 2014 study by Arjomandnejad et al. used HeLa xenografts and characterized the growth kinetics and surface markers of this model. Results indicated HeLa xenografts are hyperchromatin highly malignant epithelial cells; immunohistochemistry arrays revealed that heterotropic tumors show high expression of cytokeratins (CK) and low expression of vimentin as compared to metastatic tumors. In addition, CD34 expression was correlated to angiogenesis intensity. These results provide a comprehensive description of this highly popular cell model which can help researchers in designing investigations. One of the unique features of the HeLa cell line is that it is highly stable and can be easily cultured in the laboratory. This has made it a valuable tool for producing large quantities of proteins and other biological molecules for research and medical applications. HeLa cells have been used to produce a wide range of molecules, including vaccines, monoclonal antibodies, and recombinant proteins. The HeLa cell line (human cervical) is used to create the xenograft mouse model. The HeLa xenograft model enables researchers to study microvascular density (MVD) of anti-angiogenic therapeutic agent efficacy (such as anti-VEGF or liquiritigenin).
Download Altogen Labs HeLa Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- All cells are maintained at exponential growth until commencement of the study.
- The HeLa cells are prepped for injection by trypsinizing and collecting cells. Viability is determined by using MTT assay or trypan blue exclusion test (minimum of 98-99% cell viability). The suspension volume is then adjusted to the appropriate cell concentration.
- One million cells (in 100-150 µL) of the Matrigel/HeLa cell dilution is injected s.c. into the rear flank leg per mouse (athymic BALB/C or NOD/SCID, 11-12 week old).
- Palpation of injection sites are performed three times a week. Upon formation, tumors are measured until they an average size of 90-120 mm3 is reached.
- Post-sorting into treatment groups, compound of interest is administered following the established treatment schedule.
- Tumor measurements are taken daily, with mouse weights measured 3 times a week.
- Animals are euthanized as tumors reach 2,000 cu millimeters (or the predetermined study limit size). A necropsy and tissue collection is performed.
- All tumors are removed from the animals, weighed, and then documented via digital imaging.
- Tissues are collected by performing standard gross necropsies.
- If requested by the client, tissues/tumors can be snap frozen in liquid nitrogen, prepared for histology evaluation (10% formalin), nucleic acids isolated for genetic analysis or the tissues submersed in RNA-Later reagent.
Get Instant Quote for
HeLa Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. The dosing of the experimental compound of interest is initiated, for a staged study, when the mean tumor size reaches a specified volume (typically ~120 mm3). In an unstaged study, the dosing of the compound of interest is initiated 5-7 days after xenotransplantation. Mice are dosed once or twice a day for 28 days (or other desired study duration) via the chosen route of administration. Tumor volume (mm3) is calculated via the “(W x W x L) / 2” formula, where W is tumor width and L is tumor length.
Following options are available for the HeLa xenograft model:
- HeLa Tumor Growth Delay (TGD; latency)
- HeLa Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route
- Safety toxicology, ADME, HeLa tumor immunohistochemistry, blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 40-50 mg/kg
- Imaging studies: Fluorescence-based whole body imaging, MRI
