Comparison of Commercial Transfection Reagents: Cell line optimized transfection kits for in vitro cancer research.
by Altogen Labs, 11200 Manchaca Road, Suite 203 Austin TX 78748 USA Tel. (512) 433-6177
E-mail: info@altogenlabs.com Website: www.altogenlabs.com
Introduction
The process of in vitro transfection involves introduction of genetic material into cells and it is generally used for mammalian cells and involves non-viral methods [1]. Various therapeutic cargo molecules can be used for intracellular delivery into cancer cell lines and primary cells, including plasmid DNA (pDNA), proteins, small molecules, messenger RNA (mRNA), shall RNA such as short interfering RNA (siRNA) and microRNA (miRNA) [2]. Altogen Biosystems developed optimized transfection technologies for a specific cell line by applying expertise in combinatorial chemistry, molecular biology, and cell biology.
Transfection has been in use since the 1950s and remains a vital element in cell biology research. Transfection techniques can range from physical techniques such as electroporation to chemically mediated transfection using calcium phosphate or more advanced liposomal transfection technologies [3]. In liposomal transfection, the genetic material is contained in a liposome via mixing the material with a cationic lipid, and the liposome deposits its “cargo” into the target cell. Transfection reagents can be optimized to the target cell line and protocols for transfection can also be customized. Several advanced methodologies have emerged recently such as lipid and polymer-based carrier molecules, these compounds are capable of creating liposomes, which can fuse with the cellular membrane in order to deliver the bound RNA or DNA to the cell.
Transfection: Mechanism of Action
Although there is methodological diversity with transfection techniques, chemical transfection is the method most widely used in current laboratory research. In vitro transfection is the transient or stable introduction of exogenous molecules and genetic material, DNA or RNA, into cultured mammalian cells. Transfection reagents have cationic lipids that help in the delivery of DNA and siRNA in to the cells [4]. Cationic polymers are one of the earliest chemicals used and now cationic lipids are the most popular chemical in use today. The science behind chemical transfection remains the same however and is based upon electrostatic interactions. Essentially, negatively charged genetic material binds to positively charged reagents and this allows for the genetic material to pass through cell membranes. Cellular uptake occurs through endocytosis. Inside the cell the transfection complex enters in to the nucleus and results in the gene expression [5]. Factors that can affect the specific chemical choice depend upon the genetic material that one is transfecting, as aforementioned, but factors such as pH, cell type, and ratio of the genetic material to reagent are also critical to consider.
Transfection Methods
To introduce DNA and RNA in to cultured mammalian cells different efficient transfection methods are used and they act as powerful tool in research. They include liposome-mediated transfection, non-liposomal transfection agents (lipids and polymers), electroporation, microinjection, virus-mediated gene delivery, RNA Interference (RNAi), siRNA Transfection, high throughput siRNA screens, gene silencing (RNAi) [6-8].
In Vitro Transfection Studies
Altogen Biosystems provides different in vitro transfection reagents optimized for different cell lines such as A549 cells (lung carcinoma), DU145 cells (prostate carcinoma), MCF-7 cells (breast cancer), and HepG2 cells (hepatocellular carcinoma). Below is compiled data from several studies conducted with in vitro transfection reagents that are cell line-specific.
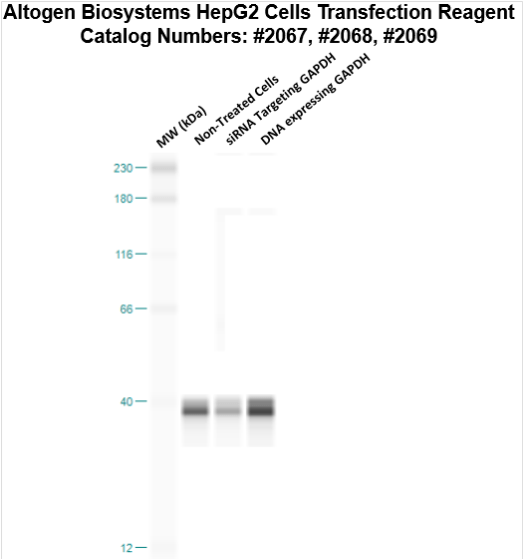
Transfection Reagent for HepG2 Cells (Hepatocellular Carcinoma Cells, HB-8065)
Figure 1. Protein expression of GAPDH in HepG2 cells. DNA plasmid expressing GAPDH or siRNA targeting GAPDH were transfected into HepG2 cells following Altogen Biosystems transfection protocol. At 72 hours post-transfection the cells were analyzed by Western Blot for protein expression levels (normalized by total protein, 10 µg of total protein loaded per each well). Untreated cells used as a negative control.
HepG2 transfection reagent advantages:
- High transfection efficiency of small RNA (siRNA, shRNA, miRNA), mRNA, pDNA
- Effective and robust intracellular delivery
- Produces consistent results, lot-to-lot, plate-to-plate, and well-to-well
- Work in the presence of serum
- A proven reagent for establishing stable cell lines
- Reagent exhibits at least 90% transfection efficiency of siRNA delivery. Transfection efficiency was determined by qRT-PCR.
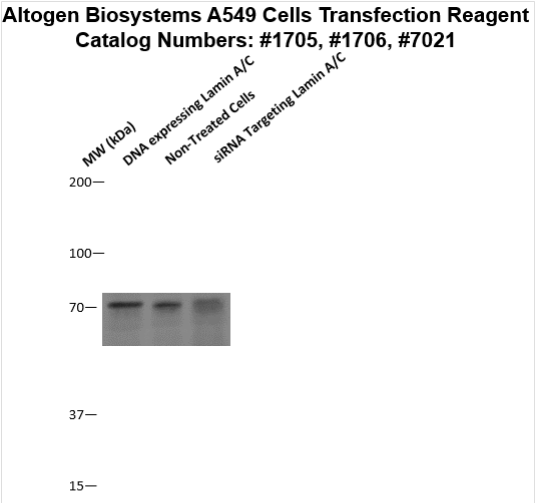
Transfection Reagent for A549 Cells (Lung Carcinoma Cells, CCL-185)
Figure 2. Protein expression of Lamin A/C in A549 cells. DNA plasmid expressing Lamin A/C or siRNA targeting Lamin A/C were transfected into A549 cells following Altogen Biosystems transfection protocol. At 72 hours post-transfection the cells were analyzed by Western Blot for protein expression levels (normalized by total protein, 10 µg of total protein loaded per each well). Untreated cells used as a negative control.
A549 transfection reagent advantages:
- Two component formulation enhances lipid mediated transfection efficiency,
- Optimized easy-to-use transfection protocol provided for transfection of RNA and DNA
- High transfection efficacy in the presence of serum
- Works well for standard reverse transfection and high-throughput applications.
- Reagent exhibits at least 80% transfection efficiency of siRNA delivery. Transfection efficiency was determined by real-time RT-PCR.
Transfection Reagent for DU145 Cells (Prostate Carcinoma Cells, HTB-81)

Figure 3. Protein expression of Lamin A/C in DU145 cells. DNA plasmid expressing Lamin A/C or siRNA targeting Lamin A/C were transfected into DU145 cells following Altogen Biosystems transfection protocol. At 72 hours post-transfection the cells were analyzed by Western Blot for protein expression levels (normalized by total protein, 10 µg of total protein loaded per each well). Untreated cells used as a negative control.
DU145 transfection reagent advantages:
- Proprietary cationic lipids formulation
- High transfection efficiency of small RNA (siRNA, shRNA, miRNA), mRNA, pDNA
- Produces consistent results, lot-to-lot, plate-to-plate, and well-to-well
- A proven reagent for establishing stable cell lines
- Optimized transfection protocols are adapted for use with both standard & reverse transfection methods.
- Reagent exhibits at least 75% transfection efficiency of siRNA delivery. Transfection efficiency was determined by qRT-PCR.
Transfection Reagent for MCF-7 Cells (Breast Cancer Cells, HTB-22)
Figure 4. Protein expression of GAPDH in MCF-7 cells. DNA plasmid expressing GAPDH or siRNA targeting GAPDH were transfected into MCF-7 cells following Altogen Biosystems transfection protocol. At 72 hours post-transfection the cells were analyzed by Western Blot for protein expression levels (normalized by total protein, 10 µg of total protein loaded per each well). Untreated cells used as a negative control.
MCF-7 transfection reagent advantages:
- Proprietary cationic lipids formulation
- High transfection efficiency of small RNA (siRNA, shRNA, miRNA), mRNA, pDNA
- Produces consistent results, lot-to-lot, plate-to-plate, and well-to-well
- Work in the presence of serum
- A proven reagent for establishing stable cell lines
- Reagent exhibits at least 85% transfection efficiency of siRNA delivery. Transfection efficiency was determined by qRT-PCR.
Transfection is a well-tested and crucial biotechnology that allows researchers to study gene products and gene functioning directly in cells. The different transfection methods allow the possibility of delivering genetic material in a very specific manner, thus making this an extremely versatile tool in preclinical biology research. Choosing the optimum method involves implementing the best transfection reagent, as well as picking the best experimental design.
Altogen Biosystems offers pre-optimized and cell line-specific in vitro transfection reagents and kits. Learn more about these transfection reagents and kits on their website and expedite your preclinical biology research study today.
www.altogen.com/products-index
Altogen Biosystems offers 100+ of cell-line specific in vitro transfection reagents.
Download full article: [Download ![]() ]
]
References:
- Hannon, G.J. and J.J. Rossi, Unlocking the potential of the human genome with RNA interference. Nature, 2004. 431(7006): p. 371-8.
- Schlake, T., et al., Developing mRNA-vaccine technologies. RNA Biol, 2012. 9(11): p. 1319-30.
- Kittler, J.T., J.G. Hanley, and J.T.R. Isaac, Transfecting and Transducing Neurons with Synthetic Nucleic Acids and Biologically Active Macromolecules
- Yin, H., et al., Non-viral vectors for gene-based therapy. Nat Rev Genet, 2014. 15(8): p. 541-55.
- Kaestner, L., A. Scholz, and P. Lipp, Conceptual and technical aspects of transfection and gene delivery. Bioorganic & medicinal chemistry letters, 2015. 25(6): p. 1171-6.
- Chang, F.H., et al., Surfection: a new platform for transfected cell arrays. Nucleic acids research, 2004. 32(3): p. e33.
- Wang, K., et al., Non-viral Delivery Systems for the Application in p53 Cancer Gene Therapy. Current medicinal chemistry, 2015. 22(35): p. 4118-36.
- Rettig, G.R. and M.A. Behlke, Progress toward in vivo use of siRNAs-II. Molecular therapy : the journal of the American Society of Gene Therapy, 2012. 20(3): p. 483-512.