Altogen Labs provides xenograft services for drug development studies of anti-cancer therapeutics ![]()
by Altogen Labs, Preclinical CRO, 11200 Manchaca Rd, Suite 203 Austin TX 78744 USA (512-433-6177)
Contact e-mail: info@altogenlabs.com
Introduction
Xenograft studies fuels the development of new therapeutics efficiently and effectively and remains the gold standard for preclinical oncology research [1]. Xenograft studies, over in vitro studies, provide a more accurate schema of both the development of a particular tumor as well as the efficacy of a drug [2]. In the preclinical phase of the development of new anti-cancer drugs and therapeutics, mouse models have been crucial to determining in vivo results for decades. Profiling a therapeutic agent can be accomplished through selecting an appropriate xenograft model, carrying out studies in different tumor types and evaluating efficacy with hematology analysis, histopathology, biomarker screening and pharmacokinetic analysis.
Xenograft services rely on use of immunocompromised small animals (laboratory mice and rats). Severe combined immune deficient (NOD/SCID) mice and nude mice are effective as proxies for human subjects.
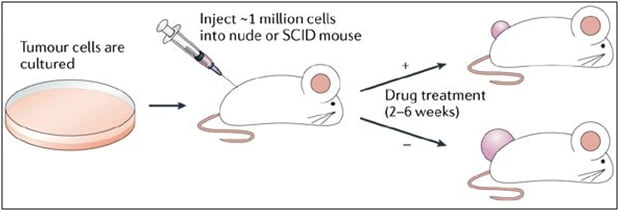
Figure 1. Study design: Xenograft and development of anti-cancer therapeutics.
Xenograft studies developed to research anti-cancer therapeutics must be rooted in technical skills such as surgical skills, general experience with drug development, thorough and efficient protocols, and most importantly, an extensive knowledge of tumors and immune-oncology. Anti-cancer therapeutic studies can investigate biomarker changes, drug efficacy, host longevity, metastases decline, delay in tumor growth and remission of tumor. Altogen Labs scientists possess years of experience and can assist with customizing the services in order to assist with designing the study and selecting the best model.
Research Study: Tumor Growth Delay in Mouse PANC-1 Xenograft Model
The goal of this study was to evaluate different test articles for efficacy against PANC-1 with the xenograft model.
Materials and methods:
Pancreas carcinoma PANC1 (CRL-1469) cell line was obtained from ATCC and cultured in ATCC-formulated Dulbecco’s Modified Eagle’s Medium (cat#302002), supplemented with fetal bovine serum to a final concentration of 10% (ATCC). Sub culturing was performed by cell trypsinization with 0.25% Trypsin-EDTA (2-3 minutes) and 1:4 split for every subsequent passage. Cell line was cultured at 37°C / 5% CO2 in humidified incubator.
Xenotransplantation (mouse xenograft model):
Immunocompromised nude mice (9- to 11-week old females) were purchased from the Harlan laboratories. All animal procedures and maintenance were conducted in accordance with the institutional guidelines. Cells were mixed (1:1 volume) with Matrigel (BD Biosciences) and cell line suspension (50% matrigel) was subcutaneously injected (1.0 x 106 cells per injection) in animal flank area to ensure successful tumor initiation and tumor growth measurements. Injection performed at respective day 0. Intravenous administration of compound (or control) started on day 7 after inoculation when measurable tumor growth was detected. Measurements of tumor volume (mm3) were performed by digital calipers every 4 days during 40 days after tumor inoculation.
| Group | # Mice | Test Article* | Total volume | Route | Dose | Injection Times |
| 1 | 10 F | No Injection | n/a | n/a | n/a | n/a |
| 2 | 10 F | Vehicle | 200ul | IV | 50 ug | Days 0, 7, 11, 14 |
| 3 | 10 F | Test Article 1 | 200ul | IV | 5 ug | |
| 4 | 10 F | Test Article 1 | 200ul | IV | 50 ug | |
| 5 | 10 F | Test Article 2 | 200ul | IV | 5 ug | |
| 6 | 10 F | Test Article 2 | 200ul | IV | 50 ug |
Table 1. Standard study design to test different test articles for efficacy against PANC-1
Animals: Athymic Nu/Nu mice
Xenograft: PANC1 (CRL-1469) cells – subcutaneous implant (flank area)
Treatment started when average tumor reached 100-150 mm3
Endpoint: Sacrifice animals when each tumor reaches 2,000 mm3 or Day 30 of the study
Services include: Gross necropsy, tumor size measurements (bi-weekly), report with statistical analysis
Results:
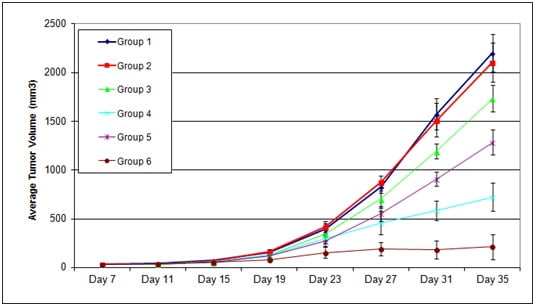
Figure 2. The capacity of Test Article 1 (TA1) and Test Article 2 (TA2) administered on Days 7, 11, 14 after tumor inoculation, was evaluated in subcutaneous PANC1 xenografts during 35 days after tumor inoculation (Day 0). Each group n=10.
Group 1 = No Injection
Group 2 = Vehicle
Group 3 = TA 1, 10 ug
Group 4 = TA 1, 50 ug
Group 5 = TA 2, 10 ug
Group 6 = TA 2, 50 ug
Conclusion
Transplanting tissue from one species to another species is truly a powerful tool; however, designing anti-cancer therapeutic studies involving xenografts also involves technical skill and experience. Altogen Labs has the knowledge, experience, and technical skills required for implementing this powerful research tool. Altogen Labs scientists can perform orthotopic, intramuscular, intravenous, intratracheal, or intraperitoneal administration.
To learn more about the extensive xenograft services that Altogen Labs offers, visit the Altogen Labs website: www.altogenlabs.com/pre-clinical-research-services/xenograft-animal-service-immunocompromised-nodscid-mice/
Altogen Labs provides all types of preclinical research and biology CRO services. Please visit our website at: www.altogenlabs.com
References:
- Ramos, P. and M. Bentires-Alj, Mechanism-based cancer therapy: resistance to therapy, therapy for resistance. Oncogene, 2015. 34(28): p. 3617-26.
- Scott, C.L., H.J. Mackay, and P. Haluska, Jr., Patient-derived xenograft models in gynecologic malignancies. Am Soc Clin Oncol Educ Book, 2014: p. e258-66.
