Melanoma originates from melanocytes, the pigment-producing cells in the skin. It is characterized by rapid metastasis and resistance to conventional treatments, making it a major challenge in oncology. Over the past few decades, significant advancements have been made in understanding the molecular mechanisms underlying melanoma progression, including the roles of mutations in genes such as BRAF, NRAS, and KIT. However, effective treatment options remain limited, particularly for advanced and metastatic melanoma. Preclinical models, particularly xenografts, have become indispensable tools for studying melanoma biology and testing new therapeutic strategies. In these models, human melanoma cells or patient-derived tumor tissues are implanted into immunodeficient mice, allowing researchers to investigate tumor growth, metastasis, and response to drugs in a biologically relevant environment. Subcutaneous, orthotopic, and patient-derived xenograft (PDX) models are commonly used to assess the efficacy of targeted therapies, immune checkpoint inhibitors, and combination treatments.
MeWo Subcutaneous, Orthotopic And Metastatic Xenograft Model: Download ![]()
MeWo cell line was derived from a patient with malignant melanoma and exhibits fibroblast-like morphology. These cells are commonly used in cancer research, particularly for studying melanoma biology and testing potential therapeutic agents. The MeWo cell line is particularly valuable for understanding the molecular mechanisms underlying melanoma progression, such as the role of genetic mutations and cellular interactions in tumor growth and metastasis. It is also used in toxicology research to assess the potential cytotoxicity of novel compounds and drug formulations. As a melanoma cell line, MeWo cells are employed in studies involving cell signaling pathways, drug resistance, and the development of targeted therapies for melanoma treatment. The cells are cultured under standard conditions and can be used in a variety of assays to evaluate cellular responses to various treatments, including chemotherapeutic agents, immunotherapies, and experimental compounds.
MeWo and the Role of AMPKα2 in Melanoma Growth and Metastasis
MeWo is characterized by genetic alterations, including mutations in the AMPKα2 (PRKAA2) gene, which frequently co-occur with NF1 mutations. Loss of AMPKα2 in MeWo cells promotes tumor growth and enhances their ability to metastasize to the brain, highlighting the importance of metabolic regulation in melanoma progression. Experimental studies show that silencing AMPKα2 increases anchorage-independent growth, a hallmark of aggressive cancer cells, and accelerates tumor expansion in vivo. The MeWo model also provides insights into the metabolic shifts that drive melanoma brain metastases, where downregulation of AMPKα2 has been linked to increased oxidative phosphorylation. Given its distinct genetic and metabolic profile, MeWo is a utilized for developing targeted therapies, particularly for NF1-mutant melanomas that currently lack effective treatment options.
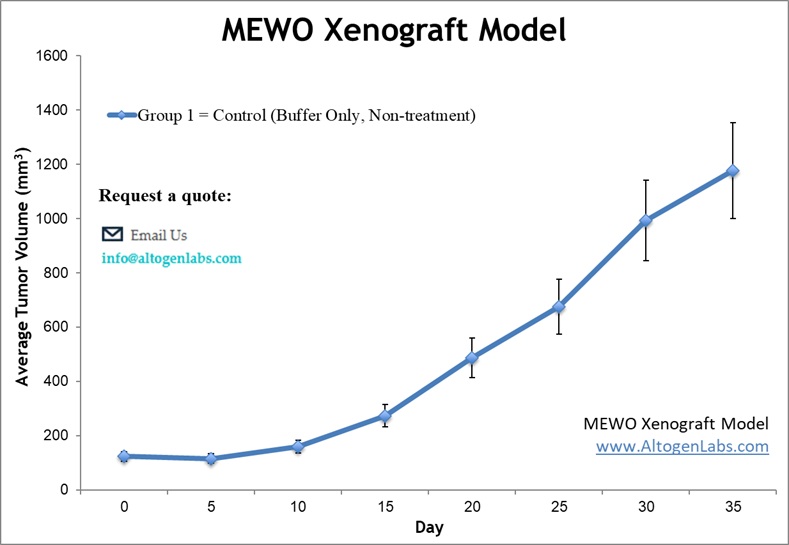
Subcutaneous MeWo Xenograft Model for Melanoma Research
The subcutaneous MeWo xenograft model is a well-established system for studying melanoma tumor growth and therapeutic response in vivo. MeWo cells, derived from a human malignant melanoma, are injected subcutaneously into immunodeficient mice, typically in the flank, allowing for consistent and measurable tumor formation. This model provides a reproducible platform for evaluating the efficacy of experimental drugs, including chemotherapeutic agents, targeted therapies, and immunotherapies. Tumor growth is monitored using digital calipers, and treatment effects are assessed based on tumor volume reduction, histological analysis, and biomarker expression. The subcutaneous model offers a straightforward approach for assessing drug efficacy, pharmacokinetics, and potential toxicities in a controlled environment. Additionally, it facilitates high-throughput screening of novel compounds before advancing to more complex orthotopic or metastatic models. This system plays a crucial role in preclinical melanoma research, aiding in the development of new treatments and improving the understanding of tumor biology.
Preclinical Evaluation of Melanoma Therapies Using Orthotopic MeWo Models
The orthotopic MeWo model is a biologically relevant system for studying melanoma growth and therapeutic response in a native microenvironment. In this model, MeWo cells are implanted intradermally or subcutaneously into the skin of immunodeficient mice, closely replicating the tumor’s natural site of origin. This approach enhances the model’s translational relevance by preserving tumor-stroma interactions, extracellular matrix composition, and vascularization patterns observed in human melanoma. Tumor progression is monitored using caliper measurements, histological analysis, and advanced imaging techniques such as bioluminescence or MRI. The orthotopic MeWo model is particularly useful for evaluating novel therapies targeting melanoma proliferation, invasion, and resistance mechanisms. Additionally, this model facilitates studies on local tumor recurrence, immune evasion, and therapeutic efficacy under conditions that better reflect clinical melanoma.
Metastatic MeWo Model for Melanoma Progression Studies
The metastatic MeWo xenograft model is a valuable system for studying melanoma progression, dissemination, and therapeutic response. MeWo cells, derived from a human malignant melanoma, have a strong propensity for metastasis when introduced into immunodeficient mice. Metastatic models are typically established by injecting MeWo cells intravenously, intradermally, or subcutaneously, leading to the formation of primary tumors and subsequent dissemination to distant organs such as the lungs, liver, and lymph nodes. This model closely mimics the metastatic cascade observed in human melanoma, making it a useful for evaluating novel anti-metastatic therapies. Researchers use advanced imaging techniques, such as bioluminescence and MRI, to track tumor spread in real time. Additionally, histological and molecular analyses of metastatic lesions provide insights into tumor heterogeneity, invasion mechanisms, and resistance pathways.
SHP2 Inhibition Suppresses MeWo Melanoma Growth and Metastasis
In a study by Zhang RY, et al., published by Oncotarget journal, researchers explored the role of SHP2 phosphatase in melanoma progression and its potential as a therapeutic target. Researchers found that SHP2 expression is significantly elevated in melanoma, correlating with increased metastasis and poorer patient prognosis. The MeWo melanoma cell line was used to investigate SHP2’s function, revealing that its overexpression enhances cell viability, motility, and anchorage-independent growth via activation of ERK1/2 and AKT signaling pathways. Conversely, SHP2 knockdown in MeWo cells reduced proliferation and induced apoptosis. Treatment with SHP2 inhibitor 11a-1 effectively blocked these pathways, leading to decreased tumor cell survival and migration. In vivo studies further confirmed that 11a-1 significantly suppresses MeWo-derived melanoma tumor growth, validating SHP2 as a promising target for melanoma therapy. These findings highlight SHP2’s pivotal role in melanoma progression and suggest that its inhibition could overcome current therapeutic limitations.
MeWo Cells Drive Adrenomedullin-Mediated Angiogenesis in Melanoma Progression
Another study by Benyahia Z, et al., published by Cancers journal, investigates the role of adrenomedullin (AM) in melanoma progression, focusing on its effects on angiogenesis and lymphangiogenesis. MeWo melanoma cells, along with A375 and SK-MEL-28 cell lines, were found to express AM and its receptors, with hypoxia further enhancing AM expression. Functional assays revealed that AM stimulates proliferation, migration, and invasion in melanoma cells, with MeWo cells exhibiting the strongest response. Blocking AM or its receptors using neutralizing antibodies significantly reduced these tumor-promoting effects. In vivo studies using MeWo xenografts demonstrated that inhibiting AM signaling leads to impaired vascularization, suppressed tumor growth, and reduced endothelial and lymphatic cell recruitment. Histological analysis confirmed the depletion of tumor vasculature and inhibition of metastasis in treated animals. These findings highlight the pivotal role of AM in melanoma development, particularly in MeWo cells, suggesting that targeting the AM system could serve as a promising therapeutic strategy for metastatic melanoma.
MITF Regulation and Apoptosis Induction in MeWo Melanoma Cells
The MeWo melanoma cell line exhibits high expression of the human antigen R (HuR) oncoprotein, which plays a crucial role in tumor progression by stabilizing oncogenic mRNAs. HuR is responsible for regulating key melanoma-associated genes, including the microphthalmia-associated transcription factor (MITF), a driver of melanoma growth and drug resistance. Studies have shown that silencing HuR via siRNA encapsulated in lipid nanoparticles significantly reduces MITF expression, leading to impaired melanoma cell proliferation and increased apoptosis. This therapeutic approach not only arrests the cell cycle in the G1 phase but also activates apoptotic pathways, including caspase-9 and PARP cleavage. Furthermore, combining HuR inhibition with MEK inhibitors synergistically enhances antitumor activity by suppressing MITF-induced drug resistance. These findings highlight HuR as a promising molecular target in MeWo melanoma cells, offering new avenues for combination therapies to improve treatment outcomes.
MeWo xenograft model serves as a valuable preclinical platform for studying melanoma progression and evaluating novel therapeutic interventions. At Altogen Labs, researchers utilize key experimental endpoints such as Tumor Growth Delay (TGD) and Tumor Growth Inhibition (TGI) to assess treatment efficacy in vivo. The MeWo cell line supports multiple administration routes, including intraperitoneal, intravenous, and subcutaneous dosing, allowing for customized therapeutic strategies. Advanced imaging techniques and tumor site-specific injections facilitate precise monitoring of tumor progression and response to treatment. Comprehensive analyses, including immunohistochemistry, molecular profiling, survival studies, and histopathological evaluations, provide critical insights into melanoma biology and mechanisms of therapy resistance. This model also enables the study of tumor-stroma interactions, angiogenesis, and metastatic potential, making it highly relevant for drug development. The MeWo xenograft model is particularly well-suited for assessing targeted therapies and immunotherapies, offering a clinically relevant system for advancing melanoma research and treatment strategies.
MeWo Subcutaneous, Orthotopic And Metastatic Xenograft Model: Download ![]()
