Validated EMT6 Allograft Tumor Model: Download ![]()
Breast cancer is the most commonly diagnosed malignancy and a leading cause of cancer-related mortality worldwide. Despite advancements in targeted therapies and immunotherapies, preclinical models remain essential for understanding tumor biology and evaluating new treatments. Allograft models, where tumor cells or tissues from the same species are transplanted into immunocompromised hosts, are often utilized in breast cancer research. Unlike xenografts, which require immunocompromised mice, allografts preserve the interaction between tumor cells and an intact immune system, making them ideal for studying immune responses and tumor microenvironment dynamics. Syngeneic models, such as EMT6 in BALB/c mice or 4T1 in the same background, allow for reproducible tumor growth while maintaining host immune surveillance. These models are particularly useful for evaluating immunotherapies, combination treatments, and metastatic progression. Additionally, allografts enable the study of tumor evolution, treatment resistance, and the impact of stromal components in a physiologically relevant setting. By closely mimicking aspects of human breast cancer in an immunocompromised system, allograft models serve as a crucial bridge between in vitro research and clinical applications.
EMT6 Cell Line
The EMT6 cell line is an epithelial cancer cell line derived from a spontaneous mammary tumor in a BALB/c mouse. It is widely used as a preclinical model for studying breast cancer due to its aggressive growth, metastatic potential, and ability to form solid tumors in syngeneic hosts. EMT6 cells exhibit rapid proliferation and can develop hypoxic regions within tumors, making them ideal for studying tumor microenvironment dynamics, radiation resistance, and immune responses. When implanted into immunocompromised BALB/c mice, EMT6 tumors closely mimic aspects of human breast cancer, particularly in terms of immune infiltration and therapeutic resistance. This model is frequently used to evaluate novel immunotherapies, chemotherapy combinations, and radiation treatments. Additionally, EMT6 tumors can metastasize to distant organs such as the lungs, providing an in vivo system for investigating metastatic progression and tumor-host interactions. Its compatibility with syngeneic models makes EMT6 valuable for assessing the effects of immune-modulating therapies in a fully functional immune system.
Altogen Labs Validated EMT6 Allograft Model
At Altogen Labs, the preclinical study design for EMT6 breast cancer models involves implanting EMT6 cells into immunocompromised BALB/c mice to evaluate tumor progression and metastasis. Female BALB/c mice, aged 6-8 weeks, are selected for tumor inoculation. To prepare the cells, EMT6 cells are cultured in DMEM with 10% FBS and antibiotics, harvested at 70-80% confluency, and resuspended at a concentration of 2 to 5 × 106 cells per 100 µL PBS for injection. A total of 2 to 5 × 106 cells are injected subcutaneously into the mammary fat pad to establish localized primary tumors. Tumor growth is monitored using caliper measurements three times per week. Once tumors reach approximately 50–100 mm³, they are surgically resected under sterile conditions. Following resection, mice are randomly assigned to treatment groups to evaluate the efficacy of immunotherapies or adjuvant treatments. Tumor tissues are analyzed via histology and immunohistochemistry to evaluate metastatic burden. Digital imaging is taken of the tumors, and remaining tissues are collected for further analysis. These tissues may be frozen in liquid nitrogen, stabilized with RNA-later reagent, submerged in 10% NBF formalin, or used to isolate nucleic acids.
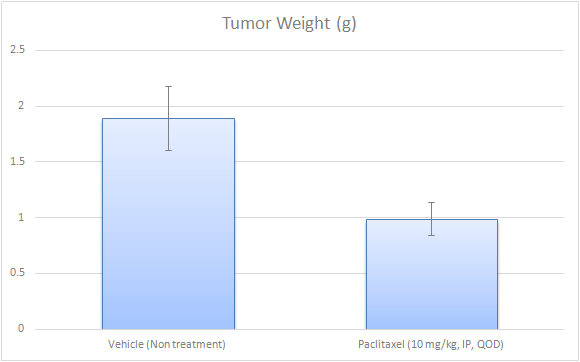
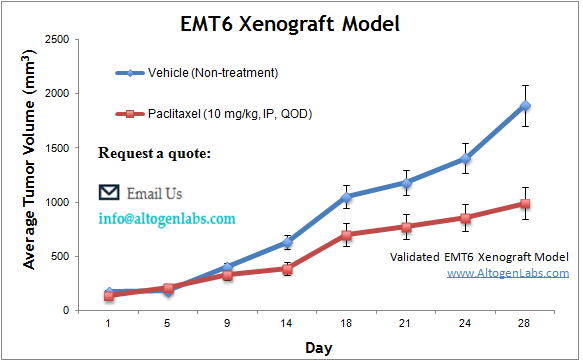
Evaluating Tumor Growth and Therapeutic Efficacy in Subcutaneous EMT6 Models
The subcutaneous EMT6 model is a widely used murine tumor model for studying breast cancer biology and therapeutic responses. In this model, EMT6 cells are injected subcutaneously, under the skin, of syngeneic BALB/c mice, typically in the hind leg region, to establish tumors that grow progressively. This model allows for easy tumor monitoring, as tumor volume can be measured externally, making it ideal for evaluating drug efficacy, immune interventions, and other therapeutic strategies. The subcutaneous EMT6 tumor is commonly used in preclinical studies to assess the impact of chemotherapy, targeted therapies, and immunotherapies. This model also provides valuable insight into tumor vasculature, hypoxia, and tumor-associated immune responses, as well as drug penetration and treatment resistance mechanisms. However, while it recapitulates many aspects of tumor growth, it does not fully mimic the tumor microenvironment or metastatic potential seen in orthotopic models. The subcutaneous EMT6 model is particularly useful for rapid screening of anti-cancer agents and understanding the biological behavior of primary breast tumors.
Oncolytic Reovirus and PD-1 Blockade as Synergistic Immunotherapy for Breast Cancer
In a study by Mostafa AA, et al., published by Cancers journal, researchers investigated the combination of oncolytic reovirus (RV) and PD-1 immune checkpoint blockade as a novel immunotherapeutic strategy for breast cancer. In vitro, RV demonstrated oncolytic activity and induced cytokine production in both human and murine breast cancer cell lines, while also upregulating PD-L1 expression. Using an EMT6 murine breast cancer model, RV monotherapy significantly reduced tumor burden and increased survival, effects that were further enhanced by PD-1 inhibition. The combination therapy promoted a robust systemic anti-tumor immune response by increasing tumor-specific CD8+ T cells and decreasing regulatory T cells in the tumor microenvironment. Additionally, treated mice exhibited long-term immunity against tumor rechallenge. These findings suggest that combining oncolytic virotherapy with immune checkpoint inhibition could be a promising strategy for breast cancer treatment and warrants further clinical investigation.
Additional Case Study: Chemerin Enhances Immune Infiltration and Suppresses Breast Tumor Growth
A study conducted by Pachynski RK, et al., published by Frontiers in Immunology, the role of chemerin (RARRES2) in suppressing EMT6 breast cancer growth by enhancing immune infiltration into the tumor microenvironment. EMT6, a murine mammary carcinoma model, was genetically engineered to overexpress chemerin, leading to significant recruitment of natural killer (NK) cells and CD8+ T cells, also known as cytotoxic T lymphocytes (CTLs), which are crucial for tumor suppression. While chemerin overexpression did not alter EMT6 tumor cell proliferation in vitro, it dramatically reduced tumor growth in vivo by promoting immune cell infiltration. Depletion experiments confirmed that both NK cells and CD8+ T cells were essential for chemerin-mediated tumor suppression, as their removal abrogated the anti-tumor effect. Additionally, chemerin expression was found to be downregulated in human breast cancer tissues, suggesting that tumors may suppress chemerin as a mechanism of immune evasion. Restoration of chemerin levels could therefore be a promising immunotherapeutic strategy to increase tumor-infiltrating lymphocytes (TILs) and enhance response to immune-based treatments. These findings position EMT6 as a valuable preclinical model for studying immune-based therapies in breast cancer.
Get Instant Quote for
EMT6 Allograft Model
Preclinical Insights into Metastatic Breast Cancer Using EMT6 Models
The metastatic EMT6 model is a widely used preclinical system to study breast cancer metastasis, particularly for evaluating therapeutic strategies targeting distant tumor spread. EMT6 cells, derived from a murine mammary carcinoma, are frequently injected into syngeneic BALB/c mice to induce spontaneous metastasis. This model enables the investigation of metastatic processes, including tumor cell migration, invasion, and colonization of distant organs such as the lungs, liver, and lymph nodes. Experimental metastasis can also be induced by intravenous or intracardiac injection of EMT6 cells, enabling the study of systemic dissemination. Researchers utilize this model to evaluate chemotherapeutic agents, immunotherapies, and combination treatments in a metastatic setting, as well as to explore tumor microenvironment interactions and immune evasion mechanisms. The model also provides insights into the efficacy of targeted therapies and the role of specific biomarkers in metastasis.
Advancing Breast Cancer Research with a Modified EMT6 Inoculation Method
The EMT6 cell line is a well-established murine model for studying breast cancer, particularly in immunocompromised BALB/c mice. Derived from a mouse mammary carcinoma, EMT6 cells exhibit aggressive tumor growth and are widely used to evaluate novel cancer therapies, including immunotherapies and radiation treatments. A modified inoculation method has been developed to improve the consistency of tumor formation by injecting EMT6 cells subcutaneously into the fourth mammary fat pad. This technique involves careful site preparation, precise needle placement, and controlled injection to ensure uniform tumor development. Tumors typically form within 5–8 days, depending on the number of cells injected, and can model both early-stage and advanced breast cancer. The modified inoculation approach also allows researchers to isolate subclinical tumor stages, providing valuable insights into tumor progression, immune interactions, and treatment responses before tumors become macroscopically visible. This model is particularly useful for evaluating localized and systemic therapies, including immunotherapy, radiotherapy, and targeted drug delivery. Because EMT6 cells are syngeneic to BALB/c mice, this system enables the study of tumor-immune interactions in a fully functional immune environment.
Evaluating Immunotherapies with Syngeneic Mouse Models
Syngeneic mouse models are critical in cancer research, particularly for investigating tumor-immune system interactions. These models involve the transplantation of tumor cells into genetically identical mice, ensuring the preservation of a functional immune system capable of mounting a natural response to the tumor. Syngeneic models are extensively utilized to evaluate immunotherapies, as they enable the study of immune responses within an intact host immune environment. They are invaluable for understanding mechanisms by which tumors evade immune surveillance and for identifying novel therapeutic targets. Additionally, these models facilitate the investigation of metastasis, the tumor microenvironment, and the effects of various treatments on immune activation. By maintaining the integrity of the host immune system, syngeneic mouse models offer more clinically relevant data for the development of cancer therapies aimed at modulating immune responses.
EMT6 Breast Cancer Growth Is Accelerated by Hyperglycemia
EMT6 is a murine mammary carcinoma cell line widely used in breast cancer research, particularly for studying tumor growth and immune interactions. Recent studies have demonstrated that hyperglycemia significantly accelerates EMT6 tumor progression by inducing pro-inflammatory pathways and promoting angiogenesis. One key mechanism involves the upregulation of miR-467, which suppresses thrombospondin-1 (TSP-1), an inhibitor of tumor angiogenesis and inflammation resolution. This leads to increased recruitment of tumor-associated macrophages (TAMs), which further enhance the inflammatory tumor microenvironment. In hyperglycemic conditions, EMT6 tumors show a marked increase in inflammatory cytokines, macrophage infiltration, and vascularization, all of which contribute to enhanced tumor growth. Interestingly, systemic inhibition of miR-467 has been shown to reduce tumor burden and TAM accumulation, suggesting a potential therapeutic strategy. Furthermore, miR-467 is detectable in blood plasma, making it a promising biomarker for breast cancer progression, particularly in diabetic patients. These findings highlight the crucial link between metabolic dysregulation, immune modulation, and EMT6 tumor growth, providing new insights into breast cancer pathophysiology and treatment.
Advancements in Organoid Technology for Personalized Cancer Research
Organoids are three-dimensional in vitro cultures derived from patient tumor samples that retain the genetic and phenotypic diversity of the original tumor, providing a more accurate representation of tumor biology compared to traditional 2D cell cultures. These models preserve complex tissue architecture and can be expanded efficiently from primary patient material, making them valuable for personalized cancer research and drug testing. Unlike xenograft and allograft models, organoids offer a faster and more scalable platform for evaluating therapeutic responses. Recent advances in organoid technology have led to the creation of patient-derived tumor organoid (PDTO) biobanks, which serve as living resources for studying cancer progression, drug resistance, and identifying potential treatments through high-throughput screening. Organoids are a often utilized in precision medicine, allowing for therapies to be tailored to individual tumor profiles while offering a bridge between cell cultures and animal models in cancer research.
Altogen Labs provides a full spectrum of services for xenotransplantation research, ensuring comprehensive support for oncology studies. Researchers can design custom study protocols to evaluate tumor growth kinetics, therapeutic efficacy, and immune response. The lab offers both subcutaneous and orthotopic allografting and xenografting techniques, with flexibility in tumor model selection based on the research needs. For host animal selection, Altogen Labs optimizes immunocompromised strains to ensure consistent and reliable results. Tumor growth is meticulously monitored, with measurements of tumor volume, body weight, and general health. Post-study services include serum collection, histological analysis, and toxicity assessments to evaluate therapeutic effects. Advanced imaging techniques, such as MRI and fluorescence-based whole-body imaging, enable real-time tracking of tumor development and treatment responses. This comprehensive approach allows Altogen Labs to support a wide range of oncology research, from drug discovery to treatment evaluation.
