Validated A673 Xenograft Tumor Model: Download ![]()
Sarcoma is a rare and diverse group of cancers that arise from connective tissues such as bone, muscle, fat, and cartilage. These tumors can occur anywhere in the body and are often aggressive, requiring extensive treatment like surgery, chemotherapy, and radiation. Studying sarcomas in the lab is challenging due to their rarity and complexity, making research models essential for understanding the disease and testing new therapies. One widely used model is the xenograft, where human sarcoma cells or tumor fragments are implanted into immunodeficient mice. These xenografts allow researchers to study tumor growth, metastasis, and drug responses in a living system that closely mimics human disease. By using xenograft models, scientists can evaluate the effectiveness of new treatments before they are tested in clinical trials. This research is crucial for improving survival rates and developing targeted therapies for patients with sarcoma.
A673 Cell Line
The A673 cell line is a human cancer cell line derived from a 15-year-old female patient diagnosed with Ewing’s sarcoma, a rare and aggressive tumor typically affecting bone or soft tissue. These cells exhibit a polygonal morphology and are commonly used in research due to their relevance in studying the molecular mechanisms underlying Ewing’s sarcoma. A673 cells are notable for harboring the EWSR1-FLI1 fusion gene, a genetic characteristic of this cancer type, making these calls a valuable model for investigating tumor biology and testing potential therapeutic strategies. This cell line has since been widely utilized in preclinical studies, including investigations into cancer progression, drug resistance, and novel targeted therapies. A673 cells grow in adherent culture and have a relatively high proliferation rate, making them suitable for in vitro experiments. Additionally, their ability to form xenografts in immunocompromised mice makes them a critical tool in in vivo studies of tumor growth and metastasis.
Altogen Labs Validated A673 Xenograft Model
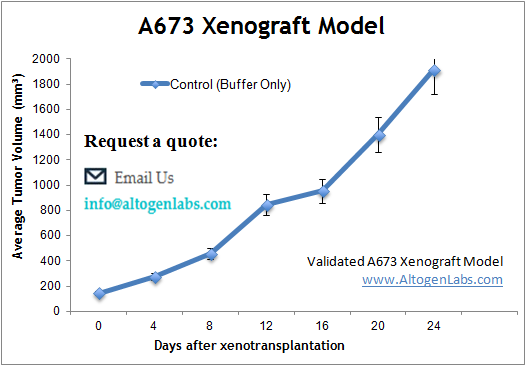
In preclinical xenograft studies at Altogen Labs, A673 cancer cells are prepared under aseptic conditions and assessed for viability (98-99%) using assays such as trypan blue exclusion or flow cytometry. The cells are then injected into the anterior flank muscle of 10-12-week-old athymic BALB/C or NOD/SCID mice, with each injection containing 1 million A673 cells mixed with Matrigel to aid in tumor establishment. Tumors are monitored for growth using digital calipers and mouse weights are recorded regularly. Once tumors reach approximately 75-150 mm³, animals are randomized into treatment groups, and tumor measurements are taken daily to assess therapeutic response. At the study’s conclusion, necropsy is performed, and tumors are excised, weighed, and analyzed histologically for tumor morphology, or for molecular analysis using RNA-later stabilization or snap freezing. Additional analyses, such as immunohistochemistry or gene expression profiling, can be conducted to evaluate therapeutic effects at the molecular level.
Efficacy of Dual VEGF-HGF Inhibition in A673 Xenografts
In a study conducted by Fiedler U, et al., published by Oncotarget journal, researchers explored the efficacy of a reference compound designed to inhibit both VEGF and HGF pathways in preclinical models, which includes A673 xenografts. The A673 cell line, a VEGF-dependent Ewing sarcoma model, was utilized to assess the reference compound’s antitumor effects. The reference compound demonstrated significant inhibition of tumor growth in A673 xenografts, achieving greater efficacy than individual VEGF or HGF inhibitors. Researchers also took a dual inhibition approach which resulted in disrupted angiogenesis and tumor proliferation that was more effective than monotherapies targeting single pathways. The reference compound also showed synergistic effects when combined with chemotherapy, enhancing antitumor activity in preclinical models. Additionally, histological analysis revealed the reference compound’s impact on vascular structure, leading to vessel regression rather than normalization.
Synergistic Effects of VEGF Blockade and Virotherapy in A673 Ewing Sarcoma
Another study by Currier MA, et al., published by Molecular Therapy journal, investigated the role of VEGF blockade in enhancing the antitumor efficacy of oncolytic herpes simplex virus (oHSV) therapy, focusing on A673 Ewing sarcoma xenografts. Despite A673 cells being highly susceptible to oHSV infection in vitro; in vivo treatment yielded only moderate tumor growth inhibition, likely due to a host inflammatory response. oHSV infection induced a neutrophilic infiltration and a reduction in tumor-associated macrophages (TAMs), accompanied by an upregulation of stroma-derived VEGF. Bevacizumab, an anti-VEGF antibody, played a crucial role in overcoming these barriers by reducing tumor angiogenesis and modulating the composition of intratumoral myeloid cells. More specifically, bevacizumab partially mitigated the depletion of tumoricidal M1 macrophages caused by oHSV, enhancing the immune-mediated antitumor response. When combined with oHSV therapy, bevacizumab significantly extended survival and induced durable tumor responses in A673 xenografted mice, outperforming either treatment alone.
Subcutaneous A673 Sarcoma Cancer Xenograft Model
The subcutaneous A673 model is a widely used xenograft model for studying Ewing sarcoma, and when implanted subcutaneously into immunodeficient mice, forms solid tumors that closely resemble the human disease. This model allows researchers to study tumor growth, angiogenesis, and response to various therapies in a controlled environment. The subcutaneous location makes it easy to monitor tumor progression and measure treatment effects non-invasively. The A673 model is particularly valuable for evaluating novel chemotherapies, targeted agents, and immunotherapies. However, it lacks the bone microenvironment seen in primary Ewing sarcoma, which may limit its ability to fully replicate the disease’s biology.
Enhancing Ewing Sarcoma Research with A673 Orthotopic Models
A673 orthotopic xenograft models involve implanting A673 cells, originally derived from Ewing’s sarcoma, into the tissue or organ that mirrors the tumor’s natural location on the immunocompromised mice. These models are particularly valuable for studying tumor growth and metastasis in a more biologically relevant tumor microenvironment compared to subcutaneous models. The orthotopic site allows the tumor to grow in its native anatomical context, making it a more accurate representation of the disease’s behavior in humans. Researchers use these models to examine tumor progression, interactions with the surrounding tissues, and to evaluate potential treatments in a more physiologically appropriate setting. Additionally, orthotopic models provide insights into the metastatic potential of the tumor, as they mimic the primary tumor microenvironment.
Modeling Ewing Sarcoma Metastasis with A673 Cells
A673 metastatic models are designed to study the spread of Ewing’s sarcoma from the primary tumor to distant organs, replicating the metastatic cascade in humans. These models are typically established by injecting A673 cells into the bloodstream or by using orthotopic injections followed by monitoring the tumor’s spread to other tissues like the lungs, liver, and bone. The development of metastatic lesions in these models provides crucial insights into the mechanisms of tumor dissemination, including cell migration, invasion, and immune evasion. Researchers use these models to evaluate the effects of potential therapeutic agents on both primary tumor growth and metastatic spread which can then be applied for preclinical drug testing that targets metastatic progression, with the goal of improving patient outcomes.
A673 Cells Oncogenic Transformation
A673, an Ewing sarcoma cell line, demonstrates oncogenic characteristics driven by the expression of the EWS/FLI oncogene. This fusion protein disrupts cell adhesion, spreading, and migration, critical processes in metastatic potential. EWS/FLI reduces the number and size of focal adhesions, diminishes actin cytoskeletal organization, and promotes a rounded cellular morphology, impairing mesenchymal features. Surprisingly, EWS/FLI inhibits rather than promotes migration and invasion, challenging conventional models of metastasis. Knockdown of EWS/FLI restores mesenchymal traits, increasing adhesion, migration, and the formation of actin stress fibers. These findings suggest that A673 cells rely on EWS/FLI to drive tumorigenesis while suppressing adhesion and motility to facilitate a passive metastatic dissemination pathway.
The A-673 xenograft model provides an excellent platform for investigating tumor progression, therapeutic efficacy, and underlying biological mechanisms in Ewing’s sarcoma. At Altogen Labs, key study options include Tumor Growth Delay (TGD) and Tumor Growth Inhibition (TGI) assessments, along with versatile dosing strategies that allow for varied frequency, duration, and routes of administration, such as intravenous, oral gavage, and intratumoral injections. Advanced analytical techniques such as immunohistochemistry, gene expression profiling, and fluorescence-based imaging for in vivo tumor monitoring are available to evaluate tumor characteristics and treatment response. Researchers can select different engraftment sites to study metastatic behavior or incorporate additional analyses such as toxicity evaluations, survival assessments, and detailed necropsy with histopathology.
