Validated A172 Xenograft Tumor Model: Download ![]()
Brain cancer is a complex and often aggressive disease characterized by uncontrolled cell growth within the brain, leading to significant neurological impairment and high mortality rates. Glioblastoma (GBM), the most common and lethal primary brain tumor, presents major therapeutic challenges due to its invasive nature and resistance to conventional treatments. To advance research and develop effective therapies, scientists rely on xenograft models, where human brain cancer cells are implanted into immunocompromised mice. Cell line-derived xenografts (CDXs), generated from established cancer cell lines, provide a reproducible and cost-effective model for studying tumor biology and drug response. Patient-derived xenografts (PDXs), on the other hand, preserve the genetic and histological characteristics of the original tumor, enhancing translational relevance. Such models are essential for testing targeted therapies, immunotherapies, and drug resistance mechanisms, ultimately contributing to the development of more effective treatments for brain cancer. The subcutaneous A172 model involves the implantation of A172 human glioblastoma cells beneath the skin of immunocompromised mice, providing a more accessible and reproducible method for preclinical studies of glioblastoma. A172 cells, originating from a human glioblastoma multiforme tumor, are injected subcutaneously into the flank of the mouse, where they form solid tumors. This model is frequently used to assess tumor growth kinetics, evaluate therapeutic responses, and test the efficacy of various treatment strategies, including small molecule inhibitors, immunotherapies, and targeted treatments. While it does not fully replicate the invasive nature of glioblastoma within the brain, the subcutaneous A172 model allows for easy tumor monitoring through caliper measurements and imaging. This model is particularly useful in early-stage drug development, offering a platform to screen for potential therapies before more complex orthotopic models are employed. Additionally, it provides valuable data on drug pharmacokinetics, systemic toxicity, and potential biomarkers for therapeutic response.
A172 Cell Line
The A172 cell line is a widely used glioblastoma model derived from the brain tissue of a 53-year-old male patient. A172 cells possess a mesenchymal gene expression profile, expressing markers such as CD90, CD105, and tenascin C, which are associated with tumor invasiveness and angiogenesis. They also secrete pro-angiogenic factors, including VEGF, FGF2(b), and TGF-β1, which contribute to tumor vascularization and progression. Comparisons with other glioblastoma lines, such as T98G, reveal differences in morphology and surface antigen expression, with both exhibiting high α2 smooth muscle actin expression. The expression of CD73 and CD105 in A172 cells is influenced by serum concentration, highlighting their adaptability to different culture conditions. Their distinct genetic and phenotypic characteristics make them a valuable tool for investigating glioblastoma pathogenesis, therapy resistance, and potential therapeutic targets.
Altogen Labs Validated A172 Xenograft Model
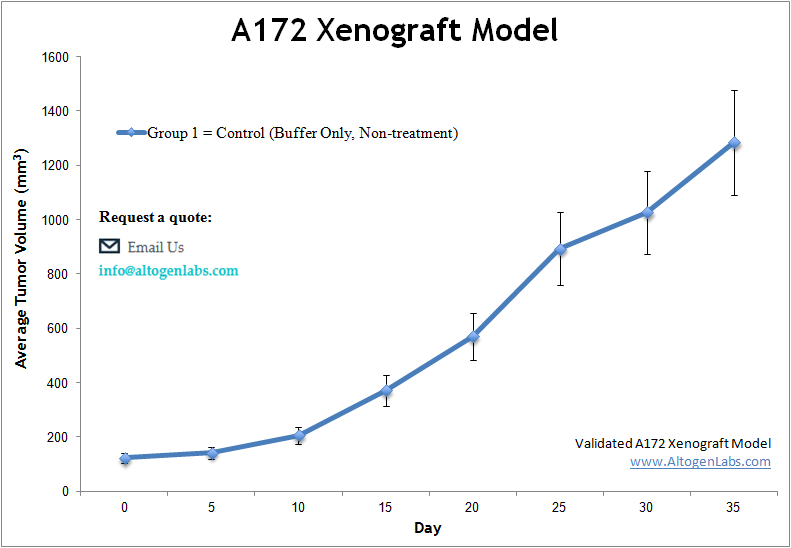
At Altogen Labs, in preclinical studies, a rigorous and well-established study design is utilized to assess the efficacy of potential treatments using athymic BALB/c nude mice and A172 tumor models. All flasks are maintained under aseptic conditions and in the exponential growth phase to ensure optimal cell health. The cells are then trypsinized for viability assessment using a trypan blue exclusion assay. A total of one million cells (injection volume = 100 µL) are inoculated into each athymic BALB/c nude mouse (10 weeks old). Each injection consists of a suspension of A172 cells mixed with Matrigel and is administered subcutaneously in the flank of the hind leg of the immunocompromised mouse. Tumor growth is monitored continuously, and once the average tumor size reaches 50-150 mm³, the animals are assigned to treatment cohorts. The injections of the compound of interest follow a dosing schedule provided by the client. Tumor measurements are taken daily using calipers, and the body weight of each mouse is recorded. Mice are euthanized once the tumors approach the predefined size limit set in the study design. Final necropsies are conducted as outlined in the termination of the experiment protocol. Immediately after tumor resection, tumors are weighed and digitally imaged. All samples collected are either frozen, prepared in 10% NBF for histology, or stabilized in RNAlater. The animals are housed in a pathogen-free animal facility, adhering to the Guide for the Care and Use of Laboratory Animals and the regulations set by the Institutional Animal Care and Use Committee (IACUC).
A172 Glioblastoma Cells and the Autophagy-Inducing Potential of PSPD3R
A study by Wang M, et al., published by Molecular Therapy Oncolytics journal, explores the anti-tumor effects of purple sweet potato delphinidin-3-rutin (PSPD3R) on glioblastoma, focusing on its impact on A172 glioma cells. PSPD3R was found to inhibit glioma cell proliferation both in vitro and in vivo, demonstrating cytostatic effects without toxicity to normal cells. Specifically, A172 cells, along with U251 cells, exhibited significant growth suppression following PSPD3R treatment, which was confirmed through viability assays and xenograft models. A key mechanism underlying this effect was PSPD3R-induced autophagy, driven by the suppression of miR-20b-5p, a microRNA known to regulate autophagy. Further analysis revealed that PSPD3R downregulated Akt and Creb phosphorylation, leading to increased expression of Atg7, an essential autophagy-related gene. Notably, experiments using miR-20b-5p inhibitors confirmed that reducing its expression enhanced autophagy in A172 cells, reinforcing its role as a crucial mediator in glioblastoma progression. The study also demonstrated that PSPD3R reduced tumor size in subcutaneous xenograft models, with high-dose treatments yielding stronger anti-tumor effects. These findings suggest that PSPD3R could serve as a promising natural compound for glioblastoma therapy by modulating the Akt/Creb/miR-20b-5p/Atg7 pathway to promote excessive autophagy and apoptosis in glioma cells, particularly in A172 cells.
Bortezomib’s Anti-Tumor Effects in A172 Glioblastoma Cells
Glioblastoma remains a highly aggressive and treatment-resistant brain cancer, requiring novel therapeutic strategies. In another study by Wang J, et al., published by the Journal of Cellular and Molecular Medicine, researchers investigated the impact of PLK4 inhibition on enhancing the anti-tumor effects of bortezomib in glioblastoma, focusing on A172 cells. The research demonstrates that PLK4 overexpression reduces the efficacy of bortezomib, while PLK4 knockdown enhances its anti-tumor effects. A172 cells, alongside other glioblastoma models, showed increased apoptosis and oxidative stress upon combined treatment with bortezomib and PLK4 inhibition. This effect was mediated through the PTEN/PI3K/AKT/mTOR signaling pathway, leading to a significant reduction in tumor proliferation and invasion. Western blot and qPCR analyses confirmed upregulation of apoptotic markers (caspase-3, Bax) and downregulation of pro-survival proteins (Bcl-2, MMPs) in A172 cells treated with the combination. In xenograft models, PLK4 suppression resulted in smaller tumor sizes, suggesting potential clinical applications. These findings indicate that targeting PLK4 in A172 glioblastoma cells enhances bortezomib’s therapeutic efficacy, providing a promising new avenue for glioblastoma treatment.
Investigating Glioblastoma with the Orthotopic A172 Mouse Model
The orthotopic A172 model involves the implantation of A172 human glioblastoma cells into the brain of immunocompromised mice to closely mimic the native tumor microenvironment. This model is widely used for studying glioblastoma due to its ability to replicate tumor growth and progression in the brain, providing a more accurate reflection of the disease compared to subcutaneous models. A172 cells are derived from a human glioblastoma multiforme (GBM) tumor, and their implantation into the brain allows for the evaluation of tumor invasiveness, angiogenesis, and response to therapeutic interventions within the context of the central nervous system (CNS). Researchers can use this model to investigate various treatment strategies, such as novel drugs, gene therapies, or immunotherapies, and assess their ability to penetrate the blood-brain barrier and reduce tumor growth. Furthermore, the orthotopic A172 model can be used to study the effects of tumor microenvironment interactions, including immune cell infiltration and blood vessel formation. With the advancement of imaging techniques, such as MRI or bioluminescence imaging, researchers can non-invasively monitor tumor progression and treatment responses over time.
HSF1 Regulates Therapy Resistance in A172 Glioblastoma Cells
The A172 glioblastoma cell line is a model system used to study glioblastoma multiforme (GBM), one of the most aggressive brain tumors. These cells exhibit cancer stem cell-like properties, contributing to therapy resistance and tumor recurrence. One of the key regulators in A172 cells is Heat Shock Factor 1 (HSF1), which plays a crucial role in promoting tumor cell survival, proliferation, and resistance to chemotherapy. HSF1 supports the maintenance of glioblastoma stem-like cells by upregulating genes such as SOX2, a marker of stemness. In sphere-forming conditions, HSF1 levels increase, enhancing the invasive and resistant characteristics of these cells. Targeting HSF1 or its downstream effector BIS (Bcl-2 interacting cell death suppressor) has been shown to sensitize A172 cells to temozolomide, a standard chemotherapeutic drug for GBM. This suggests that disrupting the HSF1-BIS axis could be a potential strategy to overcome therapy resistance. Additionally, HSF1 depletion not only reduces glioblastoma stemness but also suppresses matrix metalloprotease (MMP2) activity, limiting tumor cell invasion.
3D Chromatin Alterations Drive Oncogene Activation in A172 Glioblastoma
The A172 glioblastoma cell line exhibits significant oncogene activation driven by alterations in 3D chromatin organization. One key driver of tumorigenesis in these cells is EGFR amplification, which is associated with structural variations such as the 7p11.2 duplication. This duplication leads to the formation of novel topologically associating domains (neo-TADs) and enhancer-promoter interactions, particularly between LINC01446 and EGFR, resulting in increased EGFR expression. Additionally, chromatin loop alterations in A172 cells affect tumorigenesis-related genes, leading to the activation of oncogenes and the deactivation of tumor suppressors. Compartment switching from a transcriptionally inactive (B) to an active (A) state further contributes to the upregulation of oncogenes such as HOX gene clusters, which play a role in tumor growth and invasiveness. Structural variations, including translocations and copy number changes, further remodel chromatin conformation, enhancing oncogenic expression patterns. These findings suggest that chromatin structural alterations play a crucial role in glioblastoma pathogenesis and could be potential therapeutic targets.
Immunodeficient Mouse Models in Cancer Research
Immunodeficient mouse models are essential tools in cancer research, as they allow for the study of human tumor growth and therapeutic responses in vivo without interference from an intact immune system. These models, such as NOD-SCID, NSG, or nude mice, have a compromised or absent immune response, enabling the engraftment and growth of human tumors, including cell lines or patient-derived xenografts (PDXs). By eliminating the immune system’s ability to reject foreign cells, these mice provide a more accurate representation of human cancer biology and treatment responses. Immunodeficient mouse models are particularly valuable for evaluating the efficacy of targeted therapies, immunotherapies, and combination treatments, as they can mimic the clinical environment more closely than traditional models. Additionally, these models are widely used to investigate tumor biology, metastasis, and the interactions between human tumors and the tumor microenvironment.
The A172 xenograft model serves as a critical platform for preclinical glioblastoma research, particularly in evaluating novel therapeutic strategies targeting tumor growth, invasion, and therapy resistance. At Altogen Labs, researchers assess key experimental endpoints such as Tumor Growth Delay (TGD) and Tumor Growth Inhibition (TGI) to determine the efficacy of various treatment regimens. The A172 cell line supports multiple dosing methods, including intraperitoneal, intravenous, and intracranial injections, enabling flexible and clinically relevant therapeutic approaches. For enhanced investigation, advanced techniques such as bioluminescence imaging and MRI can be utilized to monitor tumor progression and treatment response in real time. Comprehensive analyses—including immunohistochemistry, molecular profiling, survival studies, and histopathological evaluations—provide critical insights into glioblastoma biology and treatment resistance. This model also facilitates the study of tumor angiogenesis, immune cell infiltration, and microenvironmental changes, offering a clinically relevant system for glioblastoma drug development. The A172 xenograft model is particularly valuable for investigating HSF1-mediated therapy resistance and the role of mesenchymal signaling pathways, making it an essential tool in glioblastoma research and drug discovery.
