H929 Xenograft Model
H929 cells are a human plasma cell line that was established from the bone marrow of a patient with multiple myeloma, a type of blood cancer that arises from plasma cells. H929 cells are commonly used as a model system for studying multiple myeloma and evaluating potential therapies. Plasma cell myeloma, or multiple myeloma, is defined as a cancer of plasma cells. Plasma cells are white blood cells that normally produce antibodies; symptoms of multiple myeloma include bone pain, frequent infections, anemia and bleeding. Risk factors include obesity, family history and radiation exposure. Multiple myeloma treatments include steroids, chemotherapy, stem cell transplants, thalidomide, radiation and bisphosphonates; in most cases this type of cancer is incurable. The H929 cell line was derived from the malignant effusion of a 62yo Caucasian female with multiple myeloma. The cells are of a lymphoblast morphology. The H929 model has been used in many myeloma research studies. In 2016, Vasuthasawat et al. (mAbs) published a study using H929 xenografts to investigate the combination effects of bortezomib with fusion anti-CD138-interferon α proteins in multiple myeloma. Results demonstrated that xenografted mice were successfully cured of established tumors with this antibody engineering/combination immunotherapy with bortezomib technique. In 2011, Mirandola et al. (BMC Cancer) released a study characterizing a novel biomarker, AKAP-4. The group used xenograft models, including with H929 myeloma cells, to demonstrate that tumor cells can be tract with the tumor antigen AKAP-4, which has clinical implications for therapy development monitoring. Lastly, a Blood Cancer Journal article (Seike et al. 2012) used H929 cells to characterize the combination treatment of KW-2478 with bortezomib. KW-2478 is a Hsp90 inhibitor the group previously reported, and bortezomib is a proteasome inhibitor and known myeloma treatment. Xenograft results demonstrated a synergistic enhancement of anti-tumor activity as well as a reduction of bone marrow tumor burden. Data supports the further investigation of this combination model. The H929 cell line is used to create the CDX (cell line derived) H-929 xenograft mouse model. The H929 xenograft model has been used to investigate myeloma treatments (bortezomib, anti-CD138 antibodies, KW-2478).
Basic Study Design
- H929 cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using a trypan blue exclusion assay (98% of cell viability is required). H929 cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 106 cells in 100 µL of a Matrigel-H929 cells suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 50-150 mm3 as measured via digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the pre-established treatment schedule.
- Mice weights are measured and recorded 3 times weekly; tumors are measured and recorded daily.
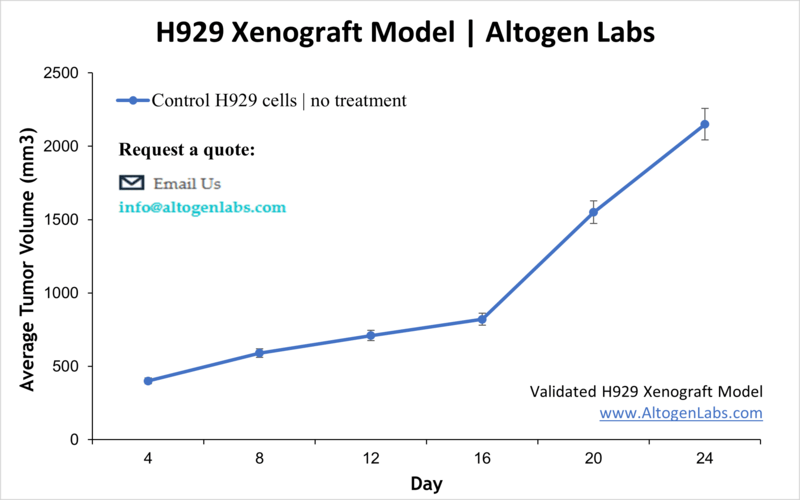
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNA-later reagent, snap frozen in LN2 or prepared for histology.
