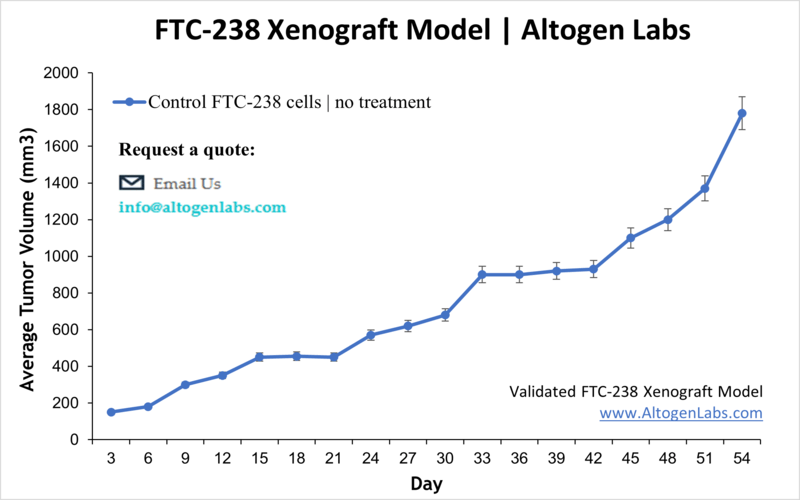
FTC-238 Xenograft Model
The thyroid gland is part of the endocrine system and secretes hormones. It is located in the neck below the Adam’s apple; thyroid cancer often has no symptoms but they may include a swelling or a neck lump. Risk factors can include radiation, other environmental factors or genetics. Thyroid carcinoma can be diagnosed with fine needle aspiration or ultrasound. The four main thyroid cancer types are: follicular, papillary, medullary and anaplastic. The FTC-238 cell line is from metastatic tissue of a 42 year old male patient with follicular thyroid carcinoma. The FTC-238 model is used in many thyroid cancer research studies. In 2016, the Endocrine Connections (Reeb et al.) article used FTC-238 cells to characterize preclinical xenograft models. The group used orthotopic models to study tumor progression and tail-vein injection models to study metastasis; while FTC-238 models developed tumors they did not develop lung metastases. Such information is important to consider when selecting appropriate models. Miao et al. (Oncology Reports, 2016) used FTC-238 cells to study the expression of ST6GalNAcII, a sialylation enzyme, and its role in cancer development. Data suggests that ST6GalNAcII mediates tumor invasion via the PI3K/Akt/NFB signaling pathways in follicular thyroid cancer (FTC). Finally, the 1994 Journal of Clinical Endocrinology Metabolism (Hoelting et al.) used the FTC 238 model to investigate the role of epidermal growth factor (EGF) on thyroid cancer. Results suggested that EGF enhances migration, invasion and proliferation of follicular (and papillary) thyroid cancer both in vitro and in vivo. The FTC-238 cell line is used to create the CDX (Cell Line Derived Xenograft) FTC-238 xenograft mouse model. The FTC-238 xenograft model has been used to study thyroid function and cancer development as well as potential treatments.
Basic Study Design
- FTC-238 cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using a trypan blue exclusion assay (98% of cell viability is required). FTC-238 cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 106 cells in 200 µL of a Matrigel-FTC238 cells suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 100-150 mm3 as measured via digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the pre-established treatment schedule.
- Mice weights are measured and recorded 2-3 times weekly; tumors are measured and recorded daily.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNAlater, snap frozen in LN2 or prepared for histology.
